Peptide Bond Definition
A peptide bond is a covalent bond formed between two amino acids. Living organisms use peptide bonds to form long chains of amino acids, known as proteins. Proteins are used in many roles including structural support, catalyzing important reactions, and recognizing molecules in the environment. A peptide bond is therefore the basis of most biological reactions. Forming peptide bonds is a requirement for all life, and the process is very similar in all forms of life.
Peptide Bond Formation
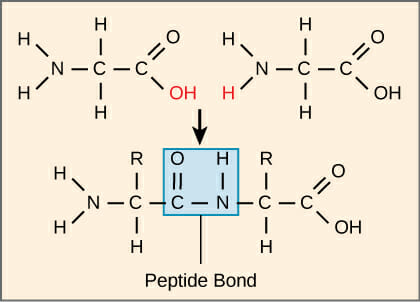
At the molecular level, a peptide bond is formed through a dehydration reaction. As seen in the image below, two amino acids are able to bond together when two hydrogens and an oxygen are removed from the molecules. One amino acid presents a carboxyl group to the reaction, and loses a hydroxyl group in the reaction (the C doubled bonded to an O). The amino group of the other amino acid loses a hydrogen. The nitrogen then substitutes in place of the hydroxyl group, forming a peptide bond. This is why peptide bonds are also known as substituted amide linkages. The two amino acids are now known as residues as they have lost several atoms and they are now covalently bonded to each other.
The carbon to nitrogen bond formed in a peptide bond is different from the carbon-nitrogen bonds in other parts of the molecule. The oxygen on the carboxyl side of the bond is slightly negative in charge. The nitrogen retains a slightly positive charge. This interaction causes the carbon and nitrogen to share more electors than they normally would, and an electric dipole is established. The extra electrons make the bond act like a double bond, which is rigid and cannot rotate. This unit of 6 molecules is known as the peptide group and is often pictured as a ball or flat plane. The carbons at the centers of each amino acid have 4 equal bonds, and can rotate freely. Thus, when many amino acids are linked together they form chains of rigid planes of atoms around the peptide bond, connected by flexible carbon bonds. This allows a peptide chain to rotate and bend, leading to the advanced formations that can catalyze reactions.
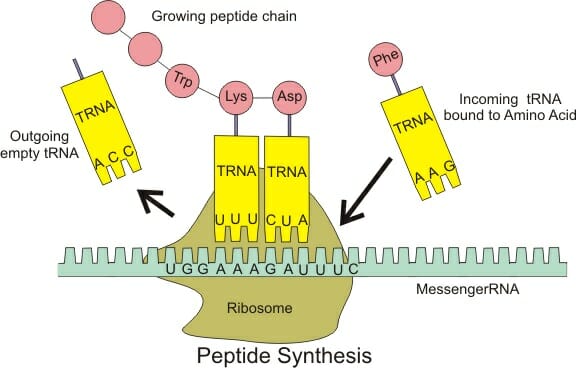
While scientist have figured out how to connect a chain of several amino acids, a typical protein has thousands of residues connected in series. Furthermore, the reaction favors individual amino acids and takes quite a bit of activation energy to do. Therefore, creating proteins without enzymes is not easy. To do this efficiently, cells have developed an efficient mechanism for building new proteins. In the genome of each organism, codons exist which describe different amino acids. The genome carries the exact sequence of these amino acids, which together will yield a functional protein. First, the information must be copied onto a messenger RNA (mRNA) molecule. Next, transfer RNAs (tRNA) binds to specific amino acids. These tRNAs correspond to different mRNA codons, which in turn correspond to different DNA codons. The actual peptide bond is formed in a special protein macrostructure known as the ribosome, pictured below.
The ribosome is a very large and complex cellular structure consisting of proteins, RNA and various other components that aid in catalyzing the formation of a peptide bond. This is known as the elongation stage of protein synthesis. The ribosome helps match tRNA to the corresponding mRNA. In turn, the RNA changes shape slightly, which catalyzes the reaction between two amino acids and expels a water molecule. The chain that is formed exits the ribosome. The ribosome, being a large protein itself, changes shape after the reaction has taken place, and moves further down the mRNA strand, starting the process over. Eventually a codon that signals the end of the protein is encountered letting the ribosome know the entire protein has been created. At this point, the mRNA and new protein will be expelled, and a new mRNA will be picked up, creating an entirely different protein.
All life is based on bonds between about 20 different amino acids, which all organisms use and modify to their own purpose. The number of different combinations is limitless, while peptide groups in proteins form peptide backbones in all proteins. The different groups attached to each amino acid cause the molecule to fold and bend into complicated structures, due to weak interactions between the molecules of different groups. Therefore, across the many millions of proteins created by different species, there exist several very similar structures that correspond to similar sequences of amino acids. Because amino acids are connected in a series with a similar direction, scientist typically draw and identify proteins starting from the amino, or nitrogen side, and going through the carboxyl terminal as a finishing point.
Quiz
1. When an organisms consumes other organisms, it must disassemble the peptide bonds between amino acids to be able to use the amino acids it its own proteins. Which of the following is required for the digestion of proteins?
A. Stomach Acid
B. Water
C. Teeth
2. A scientist needs to produce a large amount of a specific protein for medical purposes. Which of these methods would be the most successful in large-scale protein manufacturing?
A. Making individual proteins in test tubes.
B. Genetically engineering a bacteria to create the protein.
C. Collecting and purifying the protein from nature.
3. The peptide bonds between the amino acids valine and tyrosine are the same as the peptide bonds between the amino acids serine and lysine. However, even these small dipeptides behave much differently from each other in the way the overall molecule bends and folds. If the peptide bonds are the same, what causes this difference?
A. Each amino acid has a specific side-chain, which interacts with its surroundings.
B. The carbons in each amino acid are different, causing a different shape.
C. The amino groups of each amino acid cause changes in the shape.