Denature Definition
Denaturing a biological molecule refers to the loss of its three-dimensional (3-D) structure. Since molecules like proteins and DNA depend on their structure to accomplish their function, denaturation is accompanied by a loss of function. However, denaturation has no impact on the amino acid sequence of the protein itself.
Structure of Proteins
The structure of a protein can be divided into four levels – primary, secondary, tertiary and quaternary. Proteins are made of linear polymers of amino acids and this forms their primary structure. Even as the polypeptide is being synthesized on a ribosome, it starts to fold and form elements of its secondary structure. The most common features of a protein’s secondary structure are alpha helices and beta pleated sheets formed through extensive hydrogen bonding. These local structures formed by adjacent amino acids then come together to form the tertiary structure, where residues that are far removed from each other in the primary structure can come together in the same spatial region. This allows specific amino acids to be present in the active site, or for interaction with other molecules and to be supported by other parts of the protein that fold into a distinctive shape.
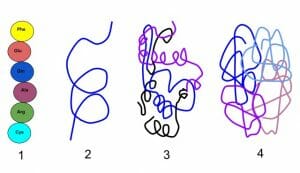
Occasionally proteins are made from more than one polypeptide. For instance, hemoglobin is a tetramer formed from four polypeptide subunits. The manner in which these subunits come together to create a functional protein forms the quaternary structure.
Except for the primary structure, the remaining three levels of protein structure are dependent mostly on non-covalent bonds such as hydrogen bonds or dipole-dipole interactions. Therefore, they are sensitive to changes in temperature, acidity, salt concentration or the presence of strong solvents. Even mild alterations to the microenvironment of a protein can lead to the loss of 3-D shape and function.
Examples of Denatured Proteins
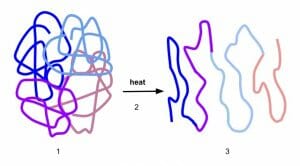
Though protein denaturation is detrimental for cell survival, it is often encountered in daily life. For instance, egg white is primarily made of soluble proteins and is liquid and translucent in fresh eggs. When it is boiled, heat denatures the proteins and makes them lose solubility. Denatured proteins aggregate and form a mass that is now opaque and solid. Similarly, altering the pH of milk by adding acids such as citric acid from lemon juice denatures milk proteins and curdles the milk. The solid white portion that separates from the whey is denatured protein. This can also be seen when milk curdles naturally due to bacterial colonization. Bacteria can produce lactic acid as a byproduct of metabolism. When properly controlled, this process of denaturation is used to make yogurt and fresh cheese.
Denatured proteins are involved in a number of diseases, from Parkinson’s disorder, Alzheimer’s to Huntington’s chorea. People suffering from these illnesses have abnormally folded proteins in some part of their body, with the brain and nervous system being particularly susceptible. Additionally, one of the major causes for blindness is the presence of denatured proteins in the lens of the eye. In this case, denaturation is said to arise due to ageing or from excess exposure to UV radiation.
Types of Denaturation
Denaturation can be classified based on the agent that causes a protein to lose its secondary, tertiary or quaternary structure. Hydrogen bonds, ionic interactions, hydrophobic interactions and Van der Waal’s forces are involved in holding these structures together. Such non-covalent interactions are disrupted by a number of factors including heat, radiation, organic solvents, acids, bases and salts because they can alter hydrophobic interactions in the interior of the protein, affect hydrogen bonding and interfere with ionic interactions.
Type I: Denaturation by change in pH
The pH of a solution has an important effect on protein structure because it changes the number and nature of hydrogen bonds and ionic interactions that take place between different amino acids. At physiological pH, many amino acid side chains are charged due to the loss or gain of a hydrogen ion.
In fact, most amino acids exist as a zwitterion within cells.
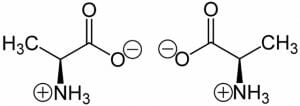
The image shows the two isomers of Alanine, where the carboxylic acid group loses a hydrogen atom and obtains a negative charge and the amine group gains a proton to become positive. The net charge on the molecule remains zero. In addition to these two charges on every amino acid, the side chain can also be polar or charged. Aspartic acid, glutamic acid, arginine, histidine and lysine are examples of amino acids that contain an extra charge at physiological conditions. However, the charge on these amino acids is dependent on pH. For instance, histidine is usually positively charged in a cell. However, it can occur in four different forms based on the pH of the solution. Due to this, it can either have a net charge of -1, 0, +1 or +2. This implies that the pH will determine the nature and number of ionic bonds a histidine residue can form with other amino acids.
When a hydrogen atom is covalently bound to a highly electronegative atom such as oxygen or nitrogen, it obtains a partial positive charge. Due to the small size of the hydrogen atom, this partial charge is sufficient to create a high charge density that can exert a strong pull on a lone pair of electrons on another atom. This attraction forms the basis of a hydrogen bond. It is sensitive to changes in pH because a change in hydrogen ion concentration can alter the nature of functional groups. For instance, amino acids such as asparagine, cysteine or tyrosine contain polar groups in their side chains. These atoms can interact with each other through hydrogen bonds based on their spatial proximity and the orientation of different side chains. However, as pH changes, these polar groups can get protonated or deprotonated, changing their ability to form hydrogen bonds. In addition, the formation of an alpha helix or a beta-pleated sheet involves hydrogen bond formation between the carboxylate and amine groups of every amino acid. If a large number of such interactions are interrupted, the protein unfolds and becomes denatured.
Finally, pH affects the overall charge of a polypeptide and this has an impact on protein solubility. If the net charge on a protein becomes zero, it can aggregate and precipitate.
Type II: Chemical Denaturation
Certain chemicals and organic solvents can cause protein denaturation. Organic solvents disrupt protein structure because most proteins sequester their hydrophobic residues towards the center of the molecule when they fold into their unique shape. Essentially, the 3-D structure of a polypeptide optimizes the energy of the molecule by lowering the interaction of hydrophobic side chains with an aqueous medium. The presence of chemicals like benzene or ethanol changes these interactions and can occasionally lead to the protein ‘flipping’ where the internal residues are presented on the outside with the loss of structure and function.
Detergents and other amphipathic molecules can also be particularly damaging. These molecules have the same effect by disrupting hydrophobic interactions and hydrogen bonds. The frothing seen during protein extraction in laboratories as well as the foam associated with household detergents are, in part, due to their denaturing effect on proteins. Heavy metals and high salt concentrations can affect the formation of ionic bonds, while chaotropic agents like urea can have an extensive disruptive effect on the hydrogen bond network.
Type III: Denaturation by Heat and Radiation
When the temperature of a biological sample is increased, it leads to an overall increase in the kinetic energy of every atom and an increase in the entropy of the system. All the atoms and molecules start to have higher energy collisions with one another as well as increased translational, rotational and vibrational motion. This reduces the strength of hydrogen bonds and weakens the influence of non-polar hydrophobic interactions. This is one of the reasons why a high fever is used by the body to fight infections. It is an attempt to retard or arrest the growth of microorganisms so that the immune system can clear the pathogens. However, a sustained high fever can be detrimental to the proteins within the host as well which is why temperatures beyond 104° Fahrenheit (40° Celsius) are considered dangerous. Prolonged exposure to radiation from the sun also leads to loss of protein structure. Sunburns and cataracts in the eye are just two examples of its deleterious effects. Radiation from other sources (such as an X-ray machine or from radioactive materials) can also cause damage to proteins and result in a number of ailments.
Functions of Denaturation
The impact of heat and radiation on proteins has its advantages as well. Food, especially meat, is cooked in order to denature the proteins within and make them easier to digest. Many packaged foods are irradiated in order to denature bacterial proteins and ensure that the food can be stored for extended periods of time. The body also secretes potent acids in the stomach in order to kill pathogens and denature proteins in food. The action of digestive enzymes is enhanced when a protein is unfolded because the peptidases gain access to all the covalent bonds holding the polypeptide together.
Additionally, denaturation is also involved in changes to the 3-D structure of nucleic acids. When the two strands of a DNA unwind, it is said to be undergoing denaturation. This type of denaturation is important for transcription and DNA replication. It is also exploited in laboratory techniques such as the polymerase chain reaction in order to produce a large number of copies of DNA in a short span of time.
Effects of Denaturation
Denaturation leads to the loss of protein function. In a protein-based enzyme, it could be a small change in the conformation of the active site, which renders it incapable of catalyzing a reaction. For proteins like antibodies, the loss of shape removes their ability to recognize and bind to antigens. When a large number of proteins within a cell are denatured, the cell undergoes severe stress in removing these molecules and synthesizing functional protein. When this process overwhelms the cell, it either undergoes apoptosis or causes disease. Due to the impact of protein denaturation on disease formation, it has been of interest to analyze whether it is possible to induce a protein to refold into its original shape after denaturation. So far, in vitro studies have only managed to achieve this reversal in a few proteins such as serum albumin, RNase and hemoglobin.
Related Biology Terms
- Amphiphile – A molecule containing hydrophilic and hydrophobic parts.
- Electronegativity – A measure of the capacity of an atom to attract a pair of electrons. Usually used in reference to a polar covalent bond where the electron cloud is skewed towards the atom that is more electronegative.
- Entropy – A measure of the degree of disorder within a system, which is related to the energy of a system unavailable for mechanical work.
- Hydrophobic Residues – Amino acids containing a hydrophobic side chain attached to the alpha carbon in addition to containing one amine and one carboxylic acid functional group.
Quiz
1. Which of these bonds is NOT commonly involved in creating the secondary or tertiary structure of a protein?
A. Hydrogen bonds
B. Covalent bonds
C. Ionic interactions
D. None of the above
2. Why does a change in pH lead to protein denaturation?
A. Alters the primary amino acid sequence
B. Alters the electronegativity of an atom
C. Changes hydrogen bond formation between various residues
D. All of the above
3. Which of these proteins has been successfully renatured?
A. Hemoglobin
B. RNase
C. Serum albumin
D. All of the above
