AP Biology 3.3 - Environmental Impacts on Enzyme Function
This section of the AP Biology curriculum focuses on the many different factors in the environment that can change how an enzyme functions. To start, we’ll do a quick review of why enzyme function is intimately related to enzyme structure. Then, we’ll start to look at factors that can affect the structure and function of enzymes. We’ll look at factors like temperature and pH that can disrupt hydrogen bonds within enzymes and cause them to denature. After this, we’ll see how the concentration of substrate molecules in a solution can also affect how quickly an enzyme functions to carry out a reaction. Finally, we’ll see how a number of molecules can serve as inhibitors and cofactors that can stop or start enzyme function and learn the difference between competitive and non-competitive (allosteric) inhibitors and cofactors.
Video Tutorial
The following video summarizes the most important aspects of this topic!
To watch more tutorial videos like this, please click here to see our full Youtube Channel!
Resources for this Standard
For Students & Teachers
- Overview & Video Tutorial (This article)
- Quick Test Prep
- Crossword Puzzle
For Teachers Only
ENDURING UNDERSTANDING
ENE-1
The highly complex organization of living systems requires constant input of energy and the exchange of macromolecules.
LEARNING OBJECTIVE
ENE-1.F
Explain how changes to the structure of an enzyme may affect its function.
ENE-1.G
Explain how the cellular environment affects enzyme activity.
ESSENTIAL KNOWLEDGE
ENE-1.F.1
Change to the molecular structure of a component in an enzymatic system may result in a change of the function or efficiency of the system –
- Denaturation of an enzyme occurs when the protein structure is disrupted, eliminating the ability to catalyze reactions.
- Environmental temperatures and pH outside the optimal range for a given enzyme will cause changes to its structure, altering the efficiency with which it catalyzes reactions.
ENE-1.F.2
In some cases, enzyme denaturation is reversible, allowing the enzyme to regain activity.
ENE-1.G.1
Environmental pH can alter the efficiency of enzyme activity, including through disruption of hydrogen bonds that provide enzyme structure.
ENE-1.G.2
The relative concentrations of substrates and products determine how efficiently and enzymatic reaction proceeds.
ENE-1.G.3
Higher environmental temperatures increase the speed of movement of molecules in a solution, increasing the frequency of collisions between enzymes and substrates and therefore increasing the rate of reaction.
ENE-1.G.4
Competitive inhibitor molecules can bind reversibly or irreversibly to the active site of the enzyme. Noncompetitive inhibitors can bind allosteric sites, changing the activity of the enzyme.
3.3 Environmental Impacts on Enzyme Function Overview
An egg is simply a big cell, packed with all sorts of enzymes and proteins that are capable of making an entirely new organism. But, when you crack an egg and place it in the heat of a frying pan, these protein enzymes start to denature. You can watch in real-time as the complex 3-D structures of these proteins denature into strings of amino acids. As the chains intertwine with each other and the water evaporates, the egg solidifies and becomes white instead of clear.
However, heat is just one environmental effect that can destroy a protein. Changes in the pH of a solution can also denature proteins, and even minor changes in the temperature or pH of a solution can affect all of the enzymes in a cell. In this video, we’re going to take a look at all of the environmental changes within a cell that can affect enzyme function. There will definitely be questions on the AP test related to this topic. So, join us as we explore how changes in the environment can impact enzyme function!
The structure and function of an enzyme are intimately related. Enzymes, like all proteins, have 4 levels of protein structure. Primary structure is formed by the sequence of amino acids in the polypeptide chain. Secondary structure is formed by interactions between adjacent amino acids that create motifs like beta-sheets and alpha-helices. Tertiary structure is formed by interactions between secondary motifs and amino acids that are created as the peptide folds back on itself. Quaternary structure is formed when several folded protein chains come together into a larger super-structure.
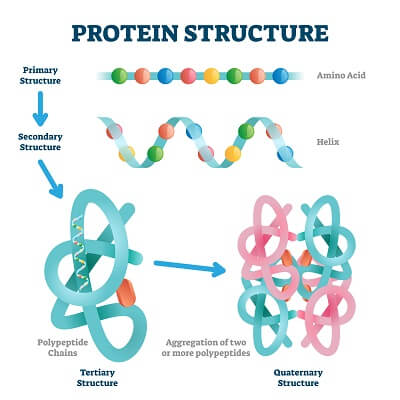
When it is working properly, an enzyme is held together into a specific shape by many different bonds at every level of the protein’s structure. In this ideal shape, the enzyme’s active site is perfectly suited to the substrate and the enzyme can easily catalyze the reaction it is supposed to. However, any disruptions to the protein’s structure can drastically alter this process. Minor alterations or broken hydrogen bonds may just mean that the enzyme becomes much less efficient than it was at ideal conditions, causing it to take much longer to process each reaction. Severe alterations in the enzyme’s structure can lead to a complete breakdown of the higher levels of protein structure – leading to a denatured enzyme that does not work at all!
Before we proceed, let’s take a step back and provide some context as to why it is important that any protein is maintained in the proper environment. Consider a protein that is used by your blood cells to transport oxygen – hemoglobin! This protein carries oxygen molecules from your lungs to your tissues. It works best at the optimal body temperature of 98.5° Fahrenheit and in a pH range of 7.6 to 7.2. Your lungs, at a pH of 7.6 and loaded with oxygen, encourage hemoglobin to pick up oxygen. The carbon dioxide in your tissues lowers the blood pH to 7.2, slightly changing the shape of hemoglobin and encouraging it to dump oxygen into the tissues. This is how hemoglobin delivers oxygen to exactly where it is needed in the body!
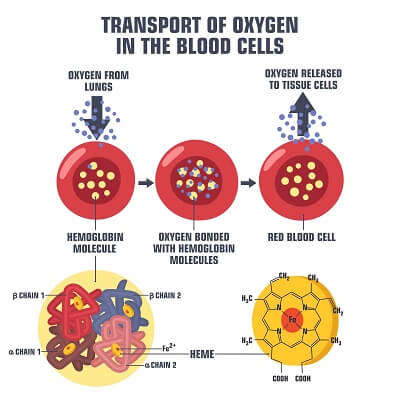
Now, think about this… what would happen if you have a severe fever or if your blood became more acidic than normal? The hemoglobin proteins in your blood cells could denature, and would not be able to carry oxygen at all! This is why it is so important that your body maintains your blood within a specific range of temperature and pH values!
Since enzymes operate like any other protein, they too are subject to changes in the environment that can affect their function. Consider a normally functioning enzyme.
It is held into a particular shape by a large number of hydrogen bonds and other interactions between amino acids and secondary motifs. These bonds hold the enzyme together, creating a functional active site that allows the enzyme to catalyze a particular reaction. If the bonds holding the enzyme in this particular conformation break down for any reason, the protein becomes denatured.
A denatured enzyme is useless because it has no functional active site. Even if some secondary motifs are still present in the structure, the enzyme has completely lost the most important parts of its 3-D shape. In rare instances with simple proteins, the enzyme can renature if it is placed back into the right environment. This means that the hydrogen bonds and weak interactions between various parts of the enzyme will reform, drawing the enzyme back into a working 3-dimensional structure. However, this is not always possible. That is why it is so important that a cell stays within a specific range of temperatures, pH values, and other environmental variables.
Furthermore, that is also the reason that complex eukaryotic cells must maintain compartmentalization by using a series of organelles. Specific enzymes within these organelles have evolved to operate within a very narrow range of environmental values. For instance, the mitochondria can get up to a blistering 122° Fahrenheit and have a pH of 7.8. The cytosol of a human cell is much closer to 98° Fahrenheit with a pH of 7.4. If all the compartments within a cell were the same pH or temperature, many of the enzymes in a cell would denature and cease to function.
To understand why temperature has such a drastic effect on enzymes and other proteins, we have to understand how temperature affects individual molecules.
Molecules are held together by bonds, and bonds are simply electrons that are shared between two atoms. Temperature is simply a way that we measure how fast the atoms in a substance are moving. If we raise the temperature, the molecules start moving faster and the individual atoms are less likely to stick together. If we lower the temperature, the atoms in a molecule slow down, come closer together, and the bonds are more rigid.
Both of these things can be detrimental to an enzyme. At high temperatures, the weak hydrogen bonds holding the enzyme in a 3-D shape start to break, and the enzyme denatures. At very low temperatures, the molecule becomes rigid and cannot undergo the necessary conformational changes it needs to in order to catalyze a reaction.
However, this does not mean that all organisms have to live in the exact same temperature range. Some bacteria that live in hot springs operate most efficiently at nearly 150° Fahrenheit, whereas some organisms that live under the icecaps thrive just below freezing. This is because the enzymes and proteins they rely on have evolved different bonds and structures that do not denature or become rigid at these extreme temperatures!

pH literally stands for “parts hydrogen”. It is essentially a measure of how much the water molecules within a solution are dissociating into the components of hydrogen and hydroxide. In a neutral solution, water molecules dissociate back and forth between hydrogen ions and hydroxide ions. If you add an acid to the solution, it causes more hydrogen ions to form within the solution and fewer hydroxide ions. This causes the solution to become more acidic. Alternatively, if you add a base to a solution, it causes the formation of more hydroxide groups and increases the pH.
Now, think about how these pH changes can change the structure and function of an enzyme. If the solution an enzyme is in becomes more acidic, there are literally more hydrogen ions within the solution. Hydrogen ions interrupt the hydrogen bonds between different parts of the enzyme, causing it to denature. On the other hand, making a solution more basic essentially does the same thing. As the charged hydroxide molecules increase in the solution, the same hydrogen bonds within the enzymes are broken and the enzyme becomes denatured. That is why it is so important for cells to maintain their pH balance within a particular range!
One of the simplest things that can affect enzyme function and the rate of the reaction is the concentration of substrate molecules within a solution. There is no doubt you will see a question on the AP test that has something to do with the substrate concentration in a solution and the reaction rate when an enzyme is present. In the most basic analysis, the reaction rate increase as the substrate concentration increases. However, you will notice that this relationship is not linear. Here’s why:
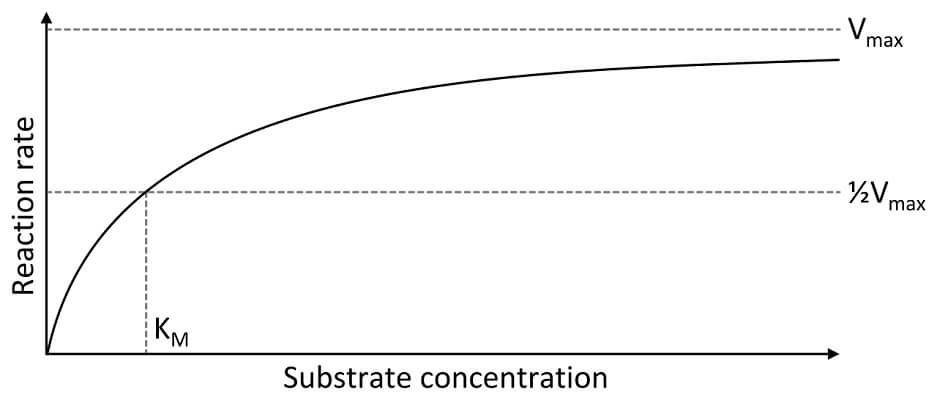
Imagine a solution of water that has a single enzyme molecule in it. To actually catalyze a reaction, the enzyme works very quickly. However, if we only put a few substrate molecules in the water, it can take the enzyme a long time to come into contact with the substrate molecules since they are being dragged around throughout the solution by hydrogen bonds. Therefore, a low substrate concentration leads to a low reaction rate.
On the other hand, the enzyme starts to reach a maximum reaction rate (known as Vmax) as the substrate concentration increases. At this concentration, it takes the enzyme very little time to find the next substrate molecule and the overall reaction can proceed as fast as the enzyme can catalyze each reaction! That is why substrate concentration can affect the rate and function of an enzyme.
Lastly, enzymes can be affected by cofactors and inhibitors – molecules that can either allow an enzyme to function in the case of a cofactor, or stop an enzyme from functioning in the case of an inhibitor. Let’s take a closer look.
Enzymes can be inhibited by a number of molecules that stop the protein from either accepting the substrate or from undergoing a conformational change to catalyze the reaction. A non-competitive or allosteric inhibitor is a molecule that binds to the enzyme in a location other than the active site. By contrast, a competitive inhibitor is a molecule that stops an enzyme from functioning by literally blocking the active site.
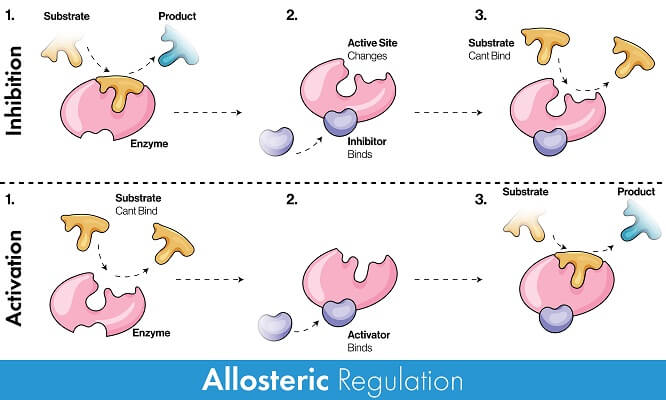
However, there are also molecules that can activate an enzyme and allow it to function properly. These are known as activators, cofactors, or coenzymes. Like inhibitors, these molecules can bind reversibly or irreversibly to the enzyme. Some cofactors and inhibitors are naturally produced by an organism to carry out a specific function, while others can be toxins found in the environment.
For example, your body uses thiamine (aka vitamin B1) to activate enzymes that help release the energy from carbohydrates. You get the coenzyme thiamine in your diet from foods like nuts. As for inhibitors, snake venom is loaded with inhibitors. One of the inhibitors actually inhibits the enzyme your body would use to detoxify the venom by breaking down proteins, allowing the venom to spread throughout your body more easily!
