AP Biology 3.2 - Enzyme Catalysis
This section of the AP Biology curriculum examines the mechanisms by which enzymes are able to catalyze biochemical reactions. We’ll start by taking a look at how reactions happen without enzymes, and why there is a certain amount of activation energy required before the reaction will take place. Then, we’ll see what happens in energetic terms to reactants as they go through a reaction catalyzed by an enzyme. To see how the enzyme actually lowers the required activation energy and speeds up a reaction, we’ll take another look at enzyme structure and the process that occurs when a substrate binds to the active site of an enzyme. Lastly, we’ll look at how enzymes work in both endothermic and exothermic reactions!
Video Tutorial
The following video summarizes the most important aspects of this topic!
To watch more tutorial videos like this, please click here to see our full Youtube Channel!
Resources for this Standard
For Students & Teachers
- Overview & Video Tutorial (This article)
- Quick Test Prep
- Crossword Puzzle
For Teachers Only
ENDURING UNDERSTANDING
ENE-1
The highly complex organization of living systems requires constant input of energy and the exchange of macromolecules.
LEARNING OBJECTIVE
ENE-1.E
Explain how enzymes affect the rate of biological reactions.
ESSENTIAL KNOWLEDGE
ENE-1.E.1
The structure and function of enzymes contribute to the regulation of biological processes —
- Enzymes are biological catalysts that facilitate chemical reactions in cells by lowering the activation energy.
3.2 Enzyme Catalysis Overview
Chemical reactions can be explosive! But, most substances do not react with each other instantaneously. Most reactions require the input of some amount of energy – called activation energy – in order to get started.
For cells, this would be a big problem. Without enzymes, cells would essentially be stuck waiting hundreds or thousands of years for certain biochemical reactions to take place. So, exactly how do enzymes lower the activation energy of a reaction? This question will definitely be on the AP test in one form or another. So, stick with us as we cover everything you need to know about enzyme catalysis!
Before we can understand how an enzyme works or why they are a necessary part of all cells, we have to understand how a typical chemical reaction takes place. Since nearly every reaction in biology takes place in an aqueous environment, let’s consider what is happening at the molecular level in a solution.
In an aqueous solution, there are trillions and trillions of water molecules all moving and pulling each other around. If we add two reactants to this solution, they will not immediately react with one another. First, the two molecules have to find each other. Given that the water molecules are dragging them around, this is no easy task. Second, the two molecules have to actually run into each other in the exact right position. If not, they may just bounce off one another. Finally, they have to be moving with enough speed in the right direction to actually push the molecules together to encourage the reaction to happen. Only then will a reaction actually occur.
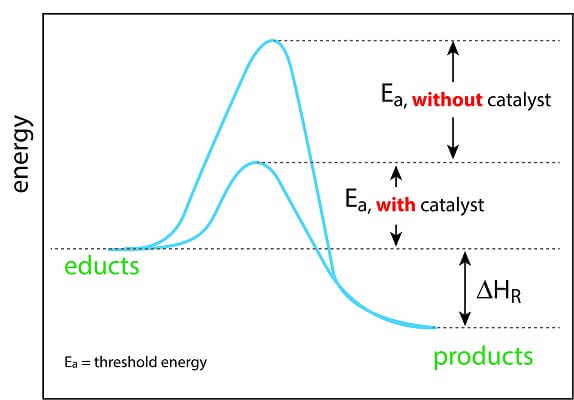
Together, all of these processes are known as “activation energy.” In a lab, we can supply the necessary activation energy by increasing the temperature, increasing the number of reactant molecules in a solution, or even by electrifying the solution. All of these methods increase the chances of two reactant molecules hitting at just the right speed and angle. However, cells do not have these advanced laboratory techniques and have to rely on enzymes. Enzymes provide the activation energy necessary for important biological reactions to happen in a timely manner.
A catalyst is any substance that speeds up the rate of a reaction, most commonly by providing the necessary activation energy for a reaction to take place. A biological catalyst – also known as an enzyme – is a protein or RNA molecule with the ability to lower the activation energy required for a specific reaction. Let’s take a closer look.
Consider a reaction where a large macromolecule is breaking down into smaller components, or monomers. Since the covalent bonds between monomers are very strong, this reaction requires quite a bit of activation energy before the reaction will start taking place. Without a catalyst, this reactant would require high temperatures or a very long time before the conditions were just right and there was enough energy supplied for the reaction to happen. Cells don’t have the patience for this!
So – over billions of years of evolution – cells have created the perfect biological machines to speed up and control important biochemical reactions. Enzymes grab the reactant, position the reactants just right, and greatly reduce the activation energy needed for a given reaction to take place.
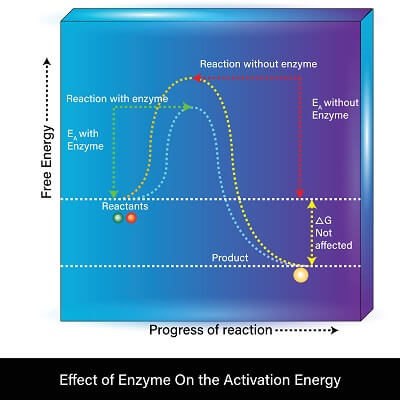
Enzymes are considered a catalyst and not a reactant of any sort for a couple of reasons. First, if we look at the product of a reaction, we can see that there is no difference in free energy between an enzyme-catalyzed reaction and a reaction that takes place without an enzyme. In other words, the product ends up with the same amount of energy with each method. The change in Gibbs Free Energy (denoted delta-G) is the same in both reactions, meaning the same amount of energy was released in the overall reaction. This shows us that the enzyme did not donate matter or energy to the reactants or products, it simply lowered the amount of energy the reactants needed to get started.
Let’s take a break from the complexity and do a quick thought experiment to help visualize how enzymes actually work. Let’s pretend that these 20 toothpicks are substrate molecules, and your fingers are an enzyme that has evolved to break toothpicks in half. Without looking at the toothpicks, you reach down, find an unbroken toothpick, and break the toothpick in half. If you do this for a minute and graph the results, you can see exactly how an enzyme works in a solution full of substrate.
At zero seconds, there are zero broken toothpicks. But, you jump right into your work and your fingers can easily find unbroken toothpicks. For a while, your work is easy and goes quickly because you can easily encounter a whole toothpick each time you reach down. Then, as you start to run out of unbroken toothpicks, your work slows. It takes your fingers longer to seek out the unbroken toothpicks and the rate of your enzyme decreases. Eventually, you run out of toothpicks to break and the rate of new products completely flattens out. This is exactly how most enzymes work in solution – they work very quickly until they have entirely run out of substrate molecules to convert into products!

So, we just finished covering what enzymes do in a biological reaction. Now, let’s take a look at how enzymes actually lower activation energy!
In an outdated model of enzyme function – called the “lock and key model” – a substrate would enter the active site, and if it fits, a reaction magically happens. If the incorrect substrate tried to enter the enzyme, the enzyme would not function and no reaction would take place. However, this model failed to explain exactly how the enzyme actually lowered the activation energy required for the reaction to take place.
The model that better explains how an enzyme actually reduces the activation energy required for a reaction to take place is called the Induced Fit model. This model starts with the same premise – that the substrate and active site must have a similar shape and chemical properties that cause an attraction. In this model, however, the substrate is not the exact perfect shape, size, or charge for the active site. As the substrate is attracted into the active site, the entire enzyme must slightly change shape to accommodate the substrate. This conformational change supplies energy to the substrate molecule, making a reaction much more likely. Let’s take a look at how this works in two different ways.
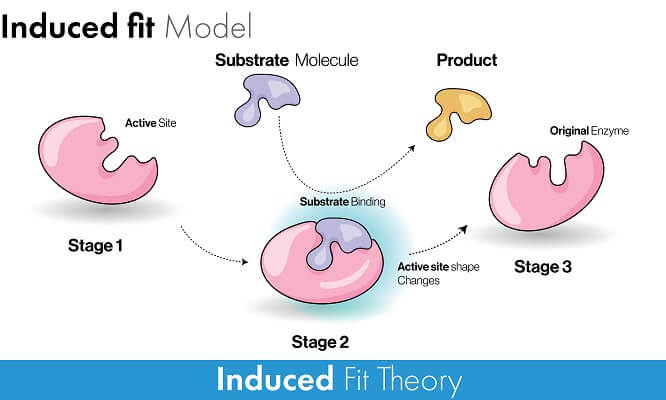
Consider an enzyme that is involved in a catabolic reaction that is breaking a substrate into smaller pieces. As the single substrate molecule binds with the enzyme, the enzyme changes shape and puts stress on a particular bond within the substrate molecule. This stress makes the bond much more likely to break – leading to the exact reaction that the enzyme is supposed to facilitate.
In an anabolic reaction that is combining two substrates into a single molecule, the opposite happens. As substrates bind to the enzyme, the enzyme changes shape to force the two molecules together. This lowers the activation energy required because the molecules are literally forced together by the enzyme and do not need to collide at the right velocity and orientation.
So, enzymes essentially reduce the activation energy required for reactions to take place by perfectly positioning substrate molecules and changing shape slightly to apply pressure where it is needed.
As we saw previously, enzymes can complete both catabolic and anabolic reactions. Since catabolic reactions break bonds, they most often release energy. Anabolic bonds typically require energy because these reactions are forming bonds. Reactions that release energy are called exothermic, while reactions that require energy from the environment are called endothermic.
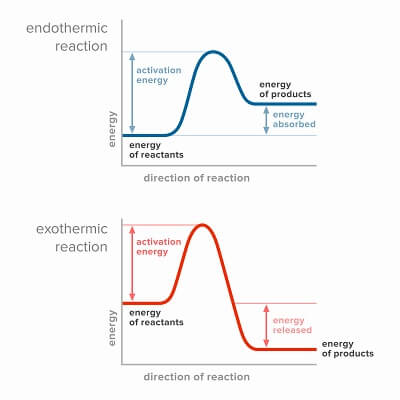
This concept sometimes confuses students on the AP test, because it would seem that if a reaction released energy, no activation energy would be required in order for the reaction to start. But, this is a misconception. Think of an exothermic reaction like a bomb. Though a ton of energy is released when the bomb goes off, it still needs an initial spark (like a lit fuse) to overcome the initial activation energy required. In most catabolic, exothermic reactions in the body, this “fuse” is an enzyme.
Likewise, students sometimes confuse activation energy with the overall energy change in a reaction. If the products of a reaction are at a higher energy level than the reactants, you may be tempted to add both the activation energy and the difference between the products and reactants to find the overall energy change. But, the overall energy change is only the difference between the reactants and products. The activation energy can easily be lowered by using an enzyme, and the overall change in energy will remain the same.
This is important because it shows that enzymes are not doing much more than bringing the right chemicals together, putting them in the right position, and stressing the formation or breakdown of the right bonds. Otherwise, enzymes affect the overall chemical reaction very little. This gives enzymes the ability to completely reset after every reaction takes place, allowing one enzyme to process hundreds of thousands of reaction cycles.
