Definition
Acetyl-CoA or acetyl coenzyme A is a component of cellular respiration (energy conversion) that adds acetyl groups to biochemical reactions. These reactions are used in the metabolizing of proteins, carbohydrates, and lipids that will provide energy sources in the forms of adenosine triphosphate (ATP), lactic acid, and ketone bodies. Recent research shows that acetyl-CoA also plays an important regulatory role in intracellular mechanisms. It also essential for energy production when fasting or starving.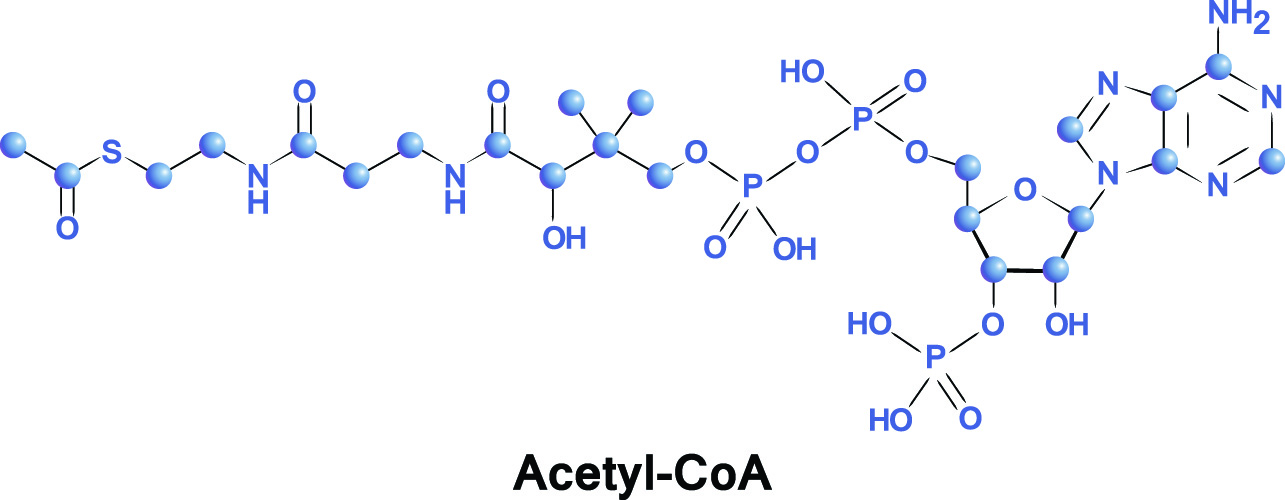
Acetyl-CoA Formation
Acetyl-CoA formation occurs inside or outside the cell mitochondria. As a metabolite (a substance necessary for metabolism), acetyl-CoA must be freely available. It can be produced via the catabolism (breakdown) of carbohydrates (glucose) and lipids (fatty acids). Its primary job is to transfer the carbon atoms in acetyl to other molecules.
The components of acetyl co-A are, not surprisingly, acetyl and coenzyme A. An acetyl group is represented by the chemical formula CH3CO. Acetyl is produced by the breakdown of pyruvate, a derivative of carbohydrate. When pyruvate breaks down, it produces small bonded carbon molecules (C2). When they react with CoA, the combined molecule becomes acetyl-CoA.
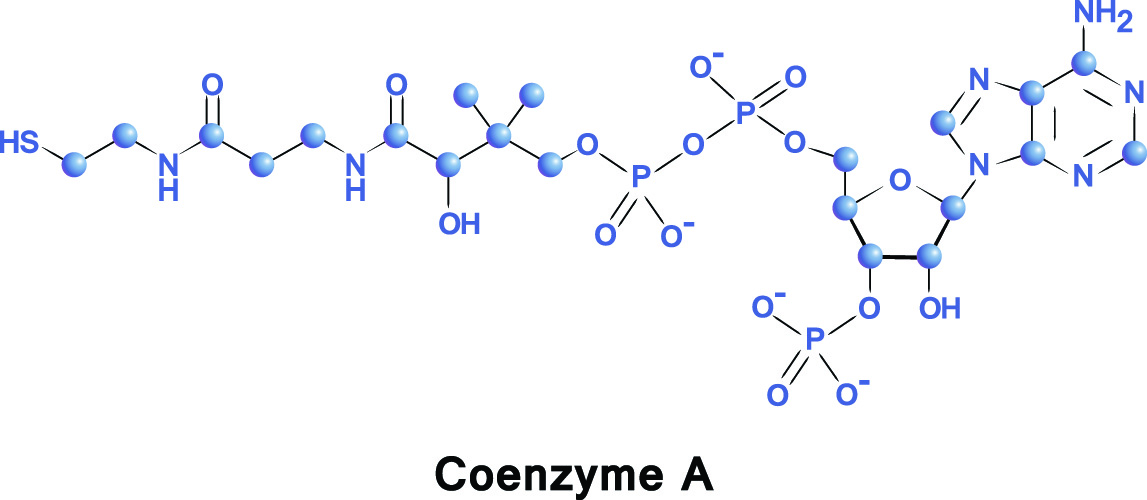
Coenzyme A is a cofactor – it assists an enzyme to provide an effect. Co-A is produced through the ingestion of vitamin B5 (pantothenic acid or pantothenate). Natural sources of this vitamin are cabbage and broccoli, whole grains, and potatoes. The chemical formula of coenzyme A is C23H38N7O17P3S.
Many types of intestinal bacteria manufacture pantothenate from certain amino acids. When pantothenate levels in the body are low, CoA and acetyl-CoA levels will also be low. As CoA production overlaps with other vitamin-producing pathways, these can also affect the availability of both CoA and acetyl-CoA. Examples of competing vitamins are folic acid and thiamine.

Acetyl binds with coenzyme A in controlled circumstances. These formation pathways are described in more detail in the following paragraphs. Basic knowledge of the Kreb’s cycle or citric acid cycle is extremely helpful when learning about acetyl-CoA.
Acetyl-CoA Formation Via Glucose
Acetyl-CoA formation most commonly occurs during glucose catabolism. After carbohydrates have been broken down by digestive enzymes, the first stage of cellular glucose metabolism or glycolysis can begin. Glycolysis is the breaking down of glucose molecules. This mechanism takes place in the cell cytosol. In the below image, glycolysis is represented in the purple box.
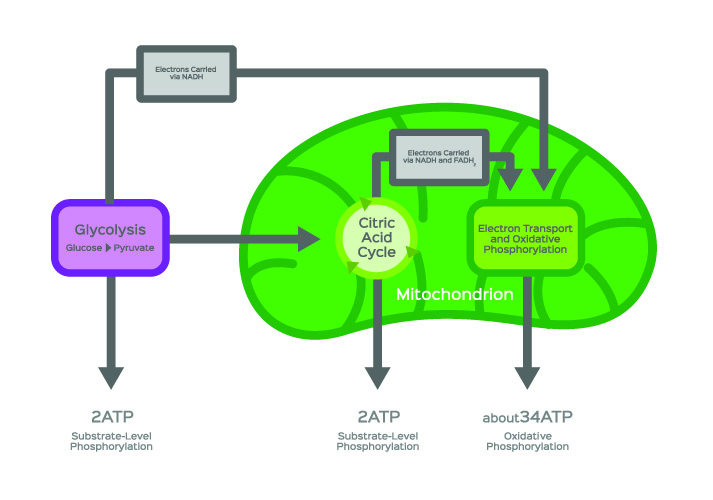
In simplified terms, a glycolysis reaction produces two hydrogen ions, a total gain of two ATP molecules, and two each of water and pyruvate molecules from a single glucose molecule (C₆H₁₂O₆).
C6 glucose becomes two C3 pyruvate molecules. The full chemical formula for pyruvate is C3H3O3 – if you look at the two chemical formulas of pyruvate and glucose, the glucose has almost been split in half. Hydrogen atoms have been released during the glycolysis reaction.
The second step of glucose metabolism depends upon the presence or absence of oxygen or the ability of the cells to use it. Where no or limited oxygen is available, pyruvate travels an anaerobic pathway that leads to lactic acid production (anaerobic respiration).

Aerobic respiration (energy production in the presence of oxygen), however, sends pyruvate into the citric acid cycle. The citric acid cycle, otherwise known as the tricarboxylic acid (TCA) cycle or Kreb’s cycle, is the foundation stone of intracellular energy production.
Entry into the aerobic cycle can only occur after three preparatory steps have taken place. Firstly, the two pyruvate molecules (C3) undergo oxidative phosphorylation (electron exchange). This step does not involve acetyl-CoA.
Secondly, an energy-releasing phase converts ADP to four ATP molecules. Again, no acetyl-CoA is required.
The third step is the conversion of pyruvate into acetyl and the subsequent binding of acetyl with available coenzyme A. Only once these three events have taken place can the next step – the Kreb’s cycle – proceed.
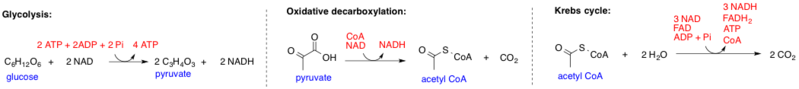
The conversion of pyruvate to acetyl-CoA is also a three-step process called pyruvate oxidative decarboxylation. This pathway takes place inside the cell mitochondria; pyruvate molecules enter the mitochondria via active transport.
First, a negatively charged carboxylate anion group (COO−) is removed from pyruvate (C3H4O3) by the enzyme pyruvate dehydrogenase to form carbon dioxide (CO2). Pyruvate has now become C2H3O or acetyl.
Second, the negative charge of the carboxylate anion group helps towards cofactor reactions (NAD+ and NADH reactions). If you are familiar with the Kreb’s cycle, you will know that these two cofactors play extremely important roles in energy production.
The final step of pyruvate oxidative decarboxylation is the bonding of coenzyme A to acetyl. This high-energy and very reactive bond forms between the acetyl group and the sulfur of coenzyme A to form acetyl-CoA. This molecule can now contribute directly to the citric acid cycle.
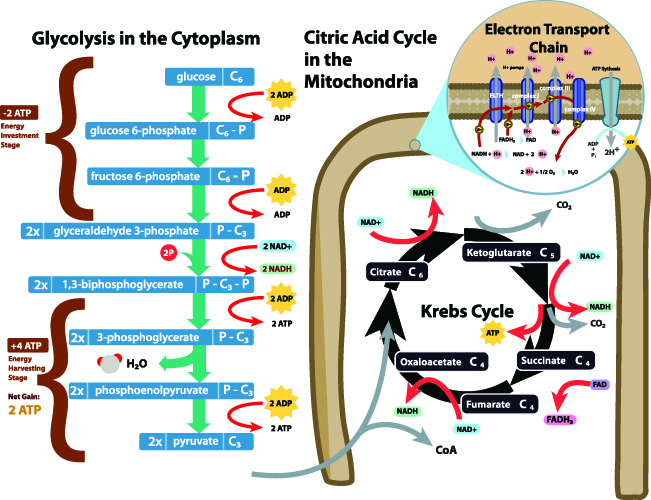
The citric acid cycle constantly forms and regenerates coenzyme A and acetyl-CoA. A single molecule of acetyl-CoA will produce 10 to 12 molecules of ATP. Where the acetyl group has been released from acetyl-CoA, the remaining coenzyme A aids in the conversion of pyruvate to acetyl CoA before re-entering the citric acid cycle.
Acetyl-CoA Formation through Fatty Acids
Acetyl-CoA formation is also said to occur via fatty acid catabolism; however, it is now understood that this acetyl-CoA is a product of carbohydrate metabolism. As acetyl-CoA can be converted into lipids and vice versa it is sometimes confused with a separate role; its true role is as a monosaccharide (glucose) metabolism catalyst.
In fat metabolism, ingested triglycerides are broken down into their smallest form – free fatty acids; these are transported into the bloodstream. Fat cells (adipocytes) in adipose tissue bind these fatty acids with glycerol and store them as triglyceride chains to serve as a backup energy source. When sources of carbohydrates are low, energy can be obtained from fat.
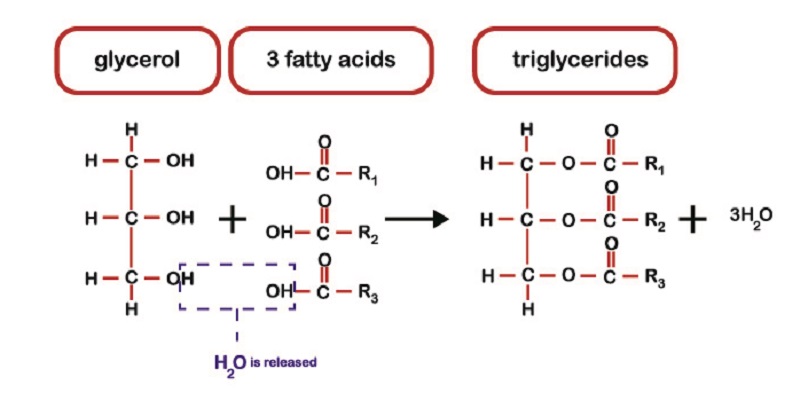
This involves lipolysis of triglycerides into negatively-charged fatty acids and glycerol. Oxidation (adding oxygen) reactions of fatty acids form fatty acyl CoA – not acetyl CoA. This is where the confusion lies. The body cannot use acyl CoA in the Kreb’s cycle. It must be converted to acetyl-CoA.
The conversion of fatty acyl CoA to acetyl CoA occurs within the mitochondria and requires the enzyme acyl CoA dehydrogenase and a whole series of reactions that continue until all of the carbons in the fatty acid chain have been converted into acetyl CoA molecules. These can then enter the Kreb’s cycle. ATP yields from fatty acids are much lower than that of monosaccharides – just 14 to carbohydrate’s 36.
Acetyl-CoA Structure
Acetyl-CoA structure is composed of a transporting coenzyme group and an attached acetyl group. A coenzyme assists an enzyme in the breakdown of a range of biological molecules.
Acetyl groups contain two carbon units and have the chemical formula C2H3O. They are composed of a methyl group (CH3) bonded via a single bond to a double-bonded carbonyl group (CO).
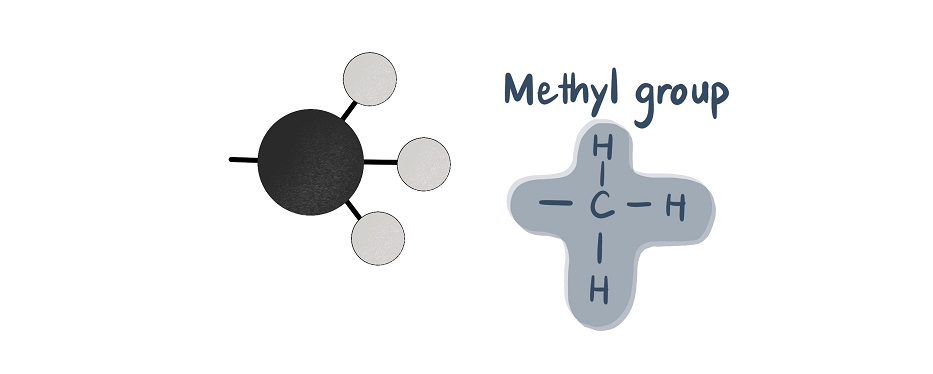
In acetyl-CoA, the acetyl group bonds to coenzyme A. Coenzyme A is a molecule composed of beta-mercaptoethylamine, pantothenic acid (an essential vitamin), phosphate, and adenosine diphosphate (ADP). The coenzyme part is a transporter for the acetyl group. It brings the acetyl group to the right place and allows the acetyl group to transfer two carbon atoms to other substances within the citric acid cycle.
Acetyl-CoA in Gluconeogenesis
Gluconeogenesis is, in simple terms, glycolysis in reverse. Where levels of glucose are low, such as in a diabetic hypoglycemic episode or during starvation or long-term fasting, the body can make glucose from non-carbohydrate sources. Acetyl-CoA plays an important regulatory role in gluconeogenesis. Most gluconeogenesis occurs in the cells of the liver; minor reactions take place in the cells of the kidneys.

In gluconeogenesis, pyruvate must first be converted to phosphoenol pyruvic (PEP) acid under the influence of several enzymes. Acetyl-CoA regulates this conversion rate as it directly controls one of the many enzymes involved in this step – pyruvate carboxylase.
Feedback concerning the body’s need for and supply of energy is also provided via acetyl-CoA availability. When acetyl-CoA levels are high, pyruvate is removed from the citric acid cycle and stored.
The next step is the conversion of fructose to a form of glucose within the endoplasmic reticulum of the (liver) cell. This glucose provides additional, cost-effective energy and also replenishes lost glycogen stores in the liver. Preparatory steps are followed as described under the Acetyl-CoA Formation Via Glucose section above.
Acetyl Coenzyme A: Additional Roles
Acetyl-CoA has many additional roles. These include lipid, cholesterol, and steroid synthesis that are the source of bile salts, sex hormones, aldosterone, and cortisol. These chemicals and hormones support a wide range of digestive, reproductive, and nervous system functions.

Ketone bodies, a popular topic of discussion in weight-loss forums, are the result of starvation events. Oxaloacetic acid availability is important within the citric acid cycle and directly associated with acetyl-CoA availability. In the citric acid cycle, acetyl-CoA combines with oxaloacetic acid to form citric acid.
When in starvation mode or during periods of hypoglycemia, glycogen reserves become depleted or cannot be used. Gluconeogenesis – glucose synthesis from fats and proteins – is necessary. If oxaloacetic acid is in short supply, acetyl CoA forms ketone bodies (ketogenesis) instead. With ketone bodies, no oxaloacetic acid is required.

It is ketone bodies that can be detected in the breath of people suffering from diabetic ketoacidosis. Ketone bodies can provide energy for the most important organs (heart, kidneys, and brain) when glucose levels are low.
This use of non-glucose energy sources is also the basis of low-carbohydrate diets such as the very low- to no-carbohydrate Atkins diet (which has caused much controversy over the years) and more recently advertised intermittent fasting lifestyles that allow carbohydrates but involve fasting states of 12 to 72-hours. The long-term effects of intermittent fasting are yet to be proven, but so far the results seem positive. Low to no carbohydrate diets seem to provide conflicting evidence. Those considering either of these diets should consult with their doctor first and arrange six-monthly blood tests.

Quiz
