A condensation reaction occurs when two molecules join to form a larger molecule and release a smaller molecule(s) in the process. The smaller molecule lost in the reaction is often water, but it can also be methanol, hydrogen chloride, acetic acid or several other molecules. Condensation reactions occur naturally in biological and chemical processes on Earth or synthetically by man-made means. If a condensation reaction happens between various parts of the same molecule, it is called intramolecular condensation. A condensation reaction that occurs between two separate molecules is called intermolecular condensation.
There are a variety of mechanisms by which condensation occurs and it depends on the chemical nature of the reactant groups and the environment in which the reaction is taking place (e.g. temperature, the presence of catalysts, functional groups, solvents used, etc.). The opposite of a condensation reaction that releases a water molecule is called a hydrolysis reaction. This happens when one molecule is split into two via the addition of a water molecule.
Examples of Condensation Reactions
Glycosylation
The basic glycosylation reaction happens when a molecule with a glycosyl group, like a carbohydrate, attaches to a functional group on another molecule. One of the most important and prevalent forms of glycosylation in nature is called N-linked glycosylation which is a post-translational process that is essential for the proper folding of proteins, matrix attachment between cells and modulating the function of proteins. In this condensation reaction, an oligosaccharide (sugar molecule) known as a glycan binds to the nitrogen atom of a protein molecule and produces a water molecule in the process.
Phosphorylation
Phosphorylation is a condensation reaction that is important for the functioning of sugars, lipids and proteins and is critical when it comes to regulating the function of enzymes. One of the simplest phosphorylation reactions in nature is the phosphorylation of glucose which is the first step in glycolysis. When glucose is phosphorylated on the 6th carbon by adenosine triphosphate (ATP) with the help of the enzyme hexokinase, the byproducts are adenosine diphosphate (ADP) and phosphoric acid.
Polypeptide and Polynucleotide Synthesis
Amino acids can condense into polypeptide molecules (proteins), releasing water as a byproduct. Also, DNA and RNA are made via polynucleotide synthesis which is also a condensation reaction. Polynucleotides form when the phosphate group on one nucleotide molecule reacts with the hydroxyl group on the carbohydrate group of another nucleotide. During this reaction, a water molecule is formed and released.
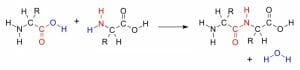
The image above shows polynucleotide synthesis using the amine group (red) of one amino acid and the carboxyl group (red) of another amino acid. The condensation reaction forms a water molecule (blue).
Nylon
Nylon is a man-made condensation polymer, meaning several identical molecular units are attached together using a condensation reaction. Nylon 66, one of the most important types of nylon, is made from adipic acid and hexamethylenediamine. The nylon polymer is formed by binding the amine groups on the hexamethylenediamine to the carboxylic acid groups on the adipic acid molecules in an alternating pattern. The byproduct of this condensation reaction is water.
The simplest form of nylon is Nylon 6 which is made from the amino acid 6-aminohexanoic acid. Water is also produced in this condensation reaction. A hydrogen molecule from the amine end of the 6-aminohexanoic acid splits off and is joined by the hydroxyl group from the carboxylic acid group at the other end of another molecule of 6-aminohexanoic acid. Then, the nitrogen and carbon atoms at the ends of each of the 6-aminohexanoic acid molecules bond to form the nylon 6 polymer.
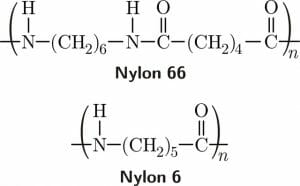
The image above shows the chemical structure of Nylon 66 and Nylon 6, two products of condensation reactions that split off water molecules.
Dacron
Dacron is the trade name for polyethylene terephthalate (PET), a man-made polyester that results from the reaction between terephthalic acid and ethylene glycol. A terephthalic acid molecule has a carboxylic acid group at each end and ethylene glycol has a hydroxyl (alcohol) group at each end. Water is formed when a carboxylic acid group and a hydroxyl group are split off. The two molecules then bind together at the ends via ester linkages.
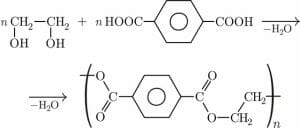
The image above shows how terephthalic acid and ethylene glycol combine to form polyethylene terephthalate via a condensation reaction that releases water as a byproduct.
References
- Condensation Reaction. (n.d.). In Wikipedia. Retrieved October 1, 2017 from https://en.wikipedia.org/wiki/Condensation_reaction
- Polymer-Condensation Polymers. (n.d.). Retrieved October 1, 2017 from http://science.jrank.org/pages/5405/Polymer.html
- Synthetic Organic Polymers. (n.d.). Retrieved October 1, 2017 from https://courses.lumenlearning.com/boundless-chemistry/chapter/synthetic-organic-polymers/
