AP Biology 4.7 - Regulation of Cell Cycle
This section of the AP Biology curriculum looks at how molecules like cyclins and cyclin-dependent kinases (CDKs) regulate the cell cycle. We’ll start with a quick review of the cell cycle. Specifically, we’ll look at how various cell cycle checkpoints can be activated and how these checkpoints are important release points for various cyclins. Then, we’ll look at how cyclins activate specific CDKs and how these CDKs can start a phosphorylation cascade that drives the cell cycle forward. After this, we’ll see how many different cyclins fluctuate in concentration throughout the entire cell cycle to activate various CDKs. In turn, this drives different parts of the cell cycle forward at the appropriate time. Finally, we’ll take a look at how mutations in CDK pathways can lead to various forms of cancer.
Video Tutorial
The following video summarizes the most important aspects of this topic!
To watch more tutorial videos like this, please click here to see our full Youtube Channel!
Resources for this Standard
For Students & Teachers
- Overview & Video Tutorial (This article)
- Quick Test Prep
- Crossword Puzzle
For Teachers Only
ENDURING UNDERSTANDING
IST-1
Heritable information provides for the continuity of life.
LEARNING OBJECTIVE
IST-1.D
Describe the role of checkpoints in regulating the cell cycle.
IST-1.E
Describe the effects of disruptions to the cell cycle on the cell or organisms.
ESSENTIAL KNOWLEDGE
IST-1.D.1
A number of internal controls or checkpoints regulate progression through the cycle.
IST-1.D.2
Interactions between cyclins and cyclin-dependent kinases.
IST-1.E.1
Disruptions to the cell cycle may result in cancer and/or programmed cell death (apoptosis).
EXCLUSION STATEMENT
Knowledge of specific cyclin-CdK pairs or growth factors is beyond the scope of the course and the AP Exam.
4.7 Regulation of Cell Cycle Overview
Regulating the cell cycle is a lot like dirt biking. At different times you must respond to a signal to slow down – like a cow in the road – while other times you can give it gas on the open trail. Your dirt bike is controlled by mechanisms at your hands and feet, while your eyes and brain respond to signals from the environment.
The cell cycle is very similar. The cell cycle either progresses or slows down based on signals from the environment and within the cell received at cell cycle checkpoints. These signals trigger signal transduction pathways that tell the cell to continue the cell cycle, slow down, or stop. Instead of your hands and feet, the signals that cells use trigger molecules like cyclins and cyclin-dependent kinase enzymes to speed up or slow down. These molecules and the concepts behind them will definitely be on the AP test. So, stick with us as we cover everything you need to know about how the cell cycle is regulated!
The cell cycle includes a number of checkpoints, regulated by signal transduction pathways, that ensure the cell cycle proceeds as planned. Let’s review the 3 most important cell cycle checkpoints.
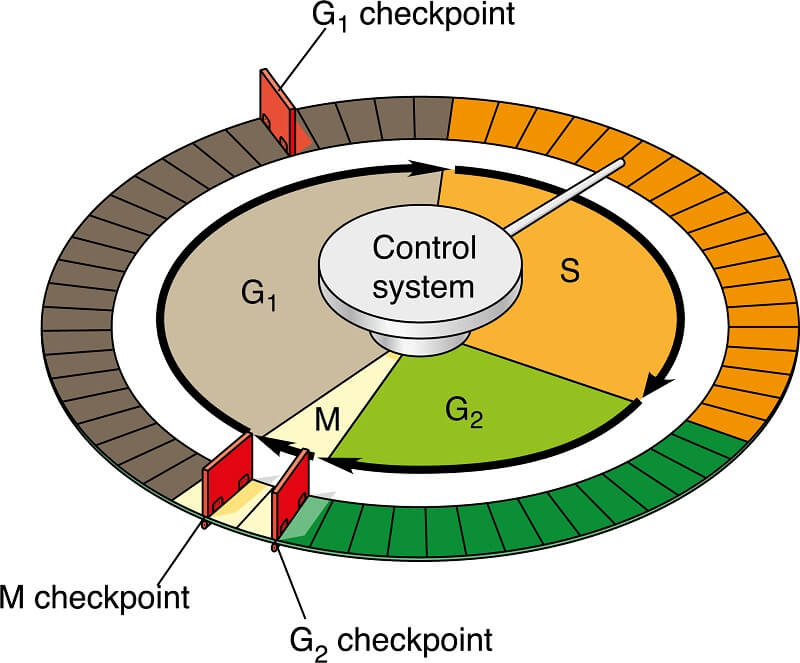
The G1 checkpoint happens right before the cell enters the S phase and replicates the DNA. During this checkpoint, the cell checks for several important things. The cell checks that it is an appropriate size to divide, that it has enough nutrients to supply both daughter cells with sufficient energy to get started, and it checks the DNA to ensure that there is no DNA damage. Each of these signals activates certain proteins, which start a cascade of signal transduction pathways that tell the cell to proceed or pause the cell cycle. If any of these conditions are not met, or if the cell receives a signal to go into quiescence, it will enter the non-dividing G0 phase.
The next checkpoint, the G2 checkpoint, comes near the end of the second growth phase. During this checkpoint, the cell ensures that DNA replication has been completed and that the DNA has not become damaged. If there is no DNA damage, the cell releases chemicals that start signal transduction pathways that lead the cell into cell division. If there has been DNA damage, the cell will enter the process of apoptosis (a.k.a. cell death) to ensure that the genetic alterations are not passed on. If this process fails, this usually means that the genes controlling the checkpoint signal transduction pathways are damaged. This is sometimes what leads to cancer cells dividing out of control!
The final checkpoint occurs during metaphase of mitosis. Sometimes called the M checkpoint and sometimes called the Spindle Checkpoint, this checkpoint takes place as the chromosomes line up on the metaphase plate. Essentially, this checkpoint ensures that the chromosomes are going to be evenly divided so both new cells have a full genetic code!
Think about this… Cancer is essentially a disease caused by a lack of regulation within the cell cycle. Theoretically, cancer is a curable disease if we can learn how to identify which cell cycle checkpoints have been corrupted, which molecules are not functioning properly, and how to synthesize and distribute those molecules to the cancer cells to stop them from dividing out of control. Keep this in mind as we start to learn the basics of how the cell cycle is regulated!
So, what are these molecules that actually control the cell cycle? The most common molecules involved in regulating the cell cycle are cyclins and cyclin-dependent kinases (CDKs).
When a cell checkpoint is passed, a signal transduction pathway is initiated that includes cyclins and CDKs. For example, if the cell cycle checkpoint is checking the DNA for damage, the enzyme that checks the DNA for damage can start the signal transduction pathway. When this enzyme finished checking the DNA, it signals a process that creates a cyclin protein.
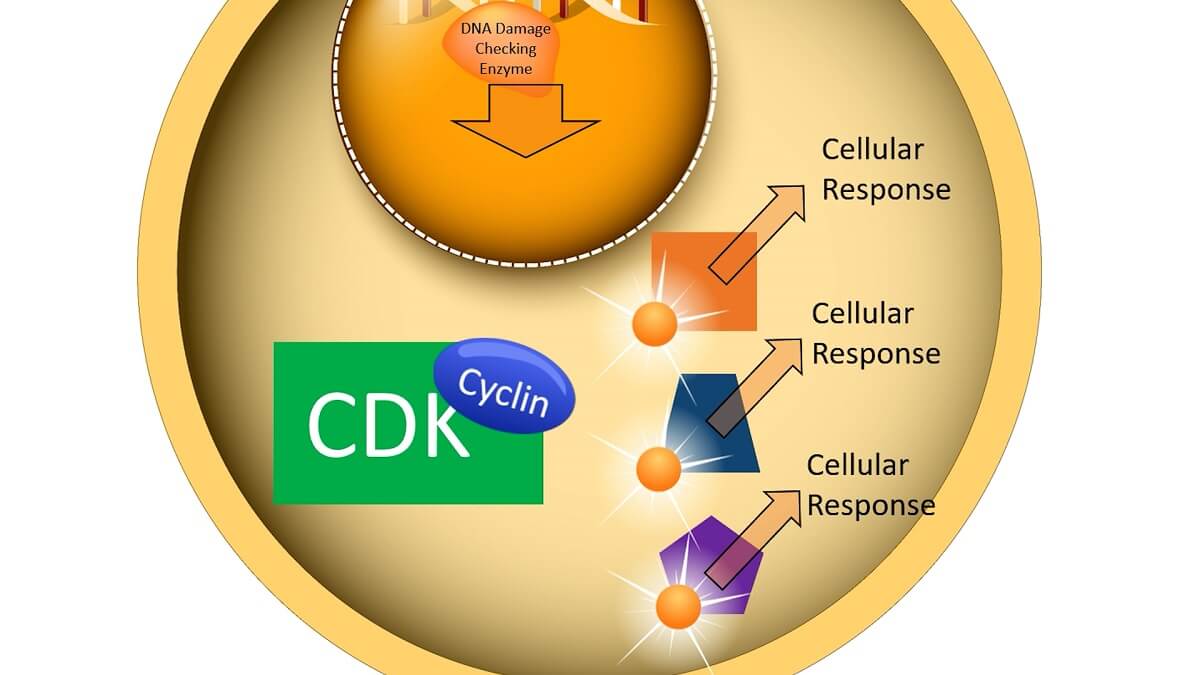
This cyclin protein seeks out a cyclin-dependent kinase molecule, most of which are always present within the cell. When a cyclin binds to the CDK, it activates a phosphorylation cascade process. The CDK becomes phosphorylated from ATP and then transfers phosphate groups to a number of other proteins and molecules within the cell. In turn, each of these proteins and molecules activates a cellular response that drives the cell cycle forward.
For example, a cyclin released during prophase activates a CDK that phosphorylates a number of other molecules. Part of this phosphorylation cascade breaks down the nuclear membrane, which allows the spindle fibers to access the chromosomes within the nucleus. Another part of this phosphorylation cascade breaks down the original cyclin, allowing a different part of the cell cycle to proceed.
While it is not necessary to memorize every cyclin, cyclin-dependent kinase, and signal transduction pathway that regulates the cell cycle for the AP Biology test, you do need to understand that there are many specific cyclins and CDKs that regulate different parts of the cell cycle. Each cyclin is released by a different cell cycle checkpoint, and each cyclin activates a specific CDK that, in turn, activates a number of cellular responses that are specific to that part of the cell cycle.
The graph below shows how the cyclins in a given organism rise and fall in concentration throughout the cell cycle. Though this graph just represents a theoretical organism, let’s take a closer look at what causes these fluctuations and how they end up regulating the cell cycle. Consider a cell entering G1 phase. As the cell starts to grow, nutrients and cell growth start signal transduction pathways that synthesize cyclin D. In turn, cyclin D activates a cyclin D-specific CDK that activates tumor suppressor genes – genes that create proteins that stop the cell from dividing prematurely.

As the cell passes the G1 checkpoint, cyclin E is created. Cyclin E activates a cyclin E-specific CDK that starts the process of DNA replication and degrades cyclin E so the replication process only happens one time.
As the cell enters the G2 phase, cyclin A peaks. Cyclin A – and the CDK specific to cyclin A – prepare the cell for cell division and starts to degrade cyclin D – the cyclin that has been activating the tumor repressor gene this whole time. With this cyclin removed, mitosis can begin. Cyclin B peaks during metaphase at the spindle checkpoint and the CDK specific to cyclin B breaks down the proteins that hold the sister chromatids together at their centromeres. This allows the cell to enter anaphase, fully separate the chromosomes, and eventually undergo cytokinesis to become 2 daughter cells. After this, the cell cycle starts anew with the slow introduction of Cyclin D in the G1 phase.
Keep in mind that these are just generalizations of cyclins and CDKs. While there are many cyclins and CDKs that carry out many of the exact same functions seen in this slide, each organism has evolved a slightly different set of cyclins and CDKs.
To fully understand the complex role that cyclins and CDKs play in the cell cycle, let’s see how cyclins can both cause cancer and potentially provide the knowledge needed to cure cancers.

Cancer, by definition, is a mass of cells that are dividing in an unregulated fashion. Unregulated cell division is undesirable because it leads to undifferentiated, mutating cells that no longer function as part of the larger organisms. Cancerous tumors can damage surrounding tissues and block important blood vessels or nerve tissues, ultimately leading to death if left untreated.
But, how do cells become cancerous in the first place? Normal cells respond to a number of signals from the organism, such as growth factors, that activate signal transduction pathways. In turn, these signal transduction pathways release cyclins. The cyclins activate CDKs, which initiate cell growth, DNA replication, and cellular division. When cells are properly listening to hormones and growth factors as cues when to divide, this leads to healthy tissues that are ordered and functional.
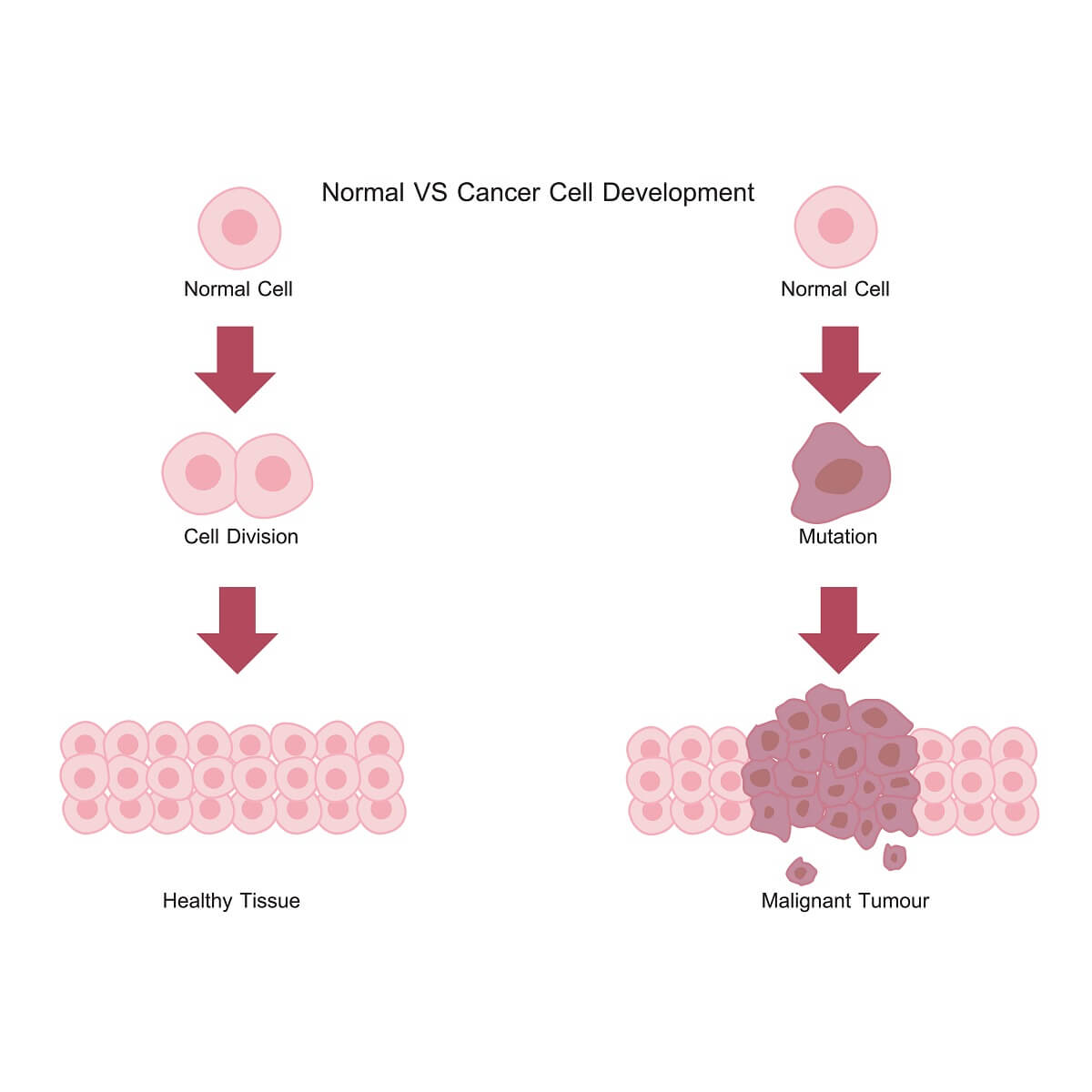
However, when a normal cell undergoes a genetic mutation that causes changes to a cyclin or CDK pathway, this can be detrimental to normal cell division. In the case of many cancers, these cells no longer require a growth factor or other hormone to activate CDK pathways. With these pathways active, the cells rapidly grow and divide – skipping important cell checkpoints and external cell signals. This is what can lead to a tumor.
But, just by knowing which cyclins and CDKs have become disrupted in a specific type of cancer, researchers can develop medicines to disrupt these pathways and shut down cell division. For example, if you have identified the CDK enzyme that is driving specific cancer, you can develop a CDK inhibitor that shuts down this enzyme and therefore stops the cell cycle. Still, many problems remain – such as how to deliver the inhibitor to only cancer cells and how to deal with cancer cells that have already created tumors.
