Tertiary Structure Definition
The tertiary structure is the structure at which polypeptide chains become functional. At this level, every protein has a specific three-dimensional shape and presents functional groups on its outer surface, allowing it to interact with other molecules, and giving it its unique function. The arrangement is made with the help of chaperones, which move the protein chain around, bringing different groups on the chain closer together in order to help them form bonds. These amino acids interacting are usually far away from each other on the chain.
The primary structure of a protein, which is the simple chain of amino acids held together by peptide bonds, is what determines the higher-order, or secondary and tertiary, structures by dictating the folding of the chain. Every amino acid has a unique side chain, or R-group, which is what gives amino acids their distinct properties.
When a protein, such as an enzyme, loses its tertiary structure, it can no longer do its job because it has become denatured and has lost its biological function. This usually happens at temperatures that are too high for the protein molecule. However, once temperatures are returned to normal, the tertiary structure can be achieved again. This suggests that it is the primary structure that is the most important for determining the more complex folding.
Tertiary Structure Interactions
The following are the main interactions that make up the tertiary structures of proteins. They guide the bending and twisting that help the protein molecule achieve a stable state. We can observe interactions that are covalent, where pairs of electrons are shared between atoms, or non-covalent, where pairs of electrons are not shared between atoms. Recall that the breaking down of these bonds can lead to the denaturation of the protein.
Hydrophobic Interactions
These non-covalent bonds are the most important factor and driving force in the formation of the tertiary structure.
If we place hydrophobic (water-hating) molecules in water, these molecules will aggregate together and form large chunks of hydrophobic molecules. Since some R-groups are hydrophilic (water-loving) and others are hydrophobic, all the amino acids containing the hydrophilic side chains, such as isoleucine, will be found on the surface of the protein, while the amino acids that have hydrophobic side chains, like alanine, will aggregate together at the center of the protein. Therefore, a protein that forms in water, as most of them do, will have a hydrophobic core and a hydrophilic surface. This is crucial in determining what the tertiary structure will look like.
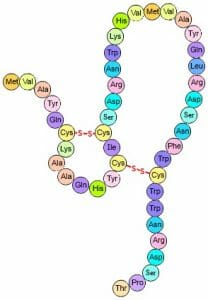
Disulfide Bridges
These are very strong covalent bonds found between cysteine residues that are in close proximity in space. The bonds form between the sulfur groups on the different cysteine residues, as shown below.
Ionic Bonds
Some amino acids contain side chains that carry positive or negative charges. If an amino acid with a positive charge comes close enough to an amino acid that carries a negative charge, they can from a bond that helps to stabilize the protein molecule.
Hydrogen Bonds
We can observe these bonds between water molecules in the solution and the hydrophilic amino acid side chains on the surface of the molecule. Hydrogen bonds also occur between polar side chains and help in stabilizing the tertiary structure.
Types of Tertiary Structures
Globular Proteins
Most proteins fall into this category. Globular proteins form a compact ball shape, where hydrophobic amino acids are found in the center of the structure and hydrophilic amino acids are found on the surface, forming a molecule that is soluble in water. Many globular proteins have domains, which are locally folded parts of the tertiary structure, ranging from 50 amino acids to 350 amino acids. One domain can be found in more than one protein if the proteins have similar functions, and a protein with multiple functions can have more than one domain, each playing a specific role. An example of globular proteins is the enzymes found within our cells.
Fibrous Proteins
Fibrous proteins are made of fibers often consisting of repeated sequences of amino acids, resulting in a highly ordered, elongated molecule. They include cartilage, which provides structural support, and are insoluble in water.
Related Biology Terms
- Cofactor – An essential non-protein component in enzymes that activates them or plays a role in the chemical reactions.
- Isomer – Compounds with different arrangements of atoms but the same chemical formula.
- Ligand – A substance, such as a hormone, that binds to a specific biomolecule to serve a purpose.
- Quaternary structure – Forms when a number of protein subunits cluster together into a complex.
Quiz
1. Which of the following is not true of the tertiary structure?
A. It is functional
B. It contains three polypeptide chains
C. It involves ionic bonds
D. It involves hydrophobic interactions
2. Which of the following refers to the sequence of amino acids?
A. Primary structure
B. Secondary structure
C. Enzyme
D. Quaternary structure
3. What dictates the arrangement of the tertiary structure?
A. The temperature that the protein is found in
B. The secondary structure of the protein
C. The amount of amino acids that make up the protein
D. The sequence of the primary structure