Deoxyribose Definition
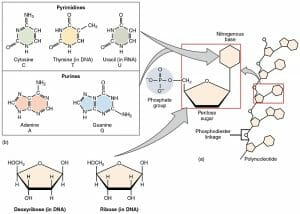
Deoxyribose is the five-carbon sugar molecule that helps form the phosphate backbone of DNA molecules. DNA, or deoxyribonucleic acid is a polymer formed of many nucleic acids. Each nucleic acid is composed of a deoxyribose molecule bound to both a phosphate group and either a purine or a pyrimidine. Purines have two carbon and nitrogen rings, while pyrimidines only have one ring. The purines are adenine (A) and guanine (G) while the pyrimidines are cytosine and thymine in DNA. In RNA, the pyrimidines are cytosine (C) and uracil (U). Connected to deoxyribose and a phosphate group, these molecules are known as deoxyribonucleotides and are the direct precursors to DNA. The bonds between nucleotides are known as phosphodiester bonds because they take place between the phosphate group of one nucleotide and the deoxyribose sugar of the next nucleotide.
Together, long strings of DNA containing many individual molecules of deoxyribose carry the genetic information of an animal. While the individual nucleotides carry no information, like a single letter, a series of three nucleotides creates a codon, which calls for a particular amino acid. Together, many amino acids form functional proteins, which can aid the cell in speeding up certain reactions. Although the deoxyribose base does not change from one nucleotide to the next, it creates a strong support for the working molecules of DNA. The only difference between RNA and DNA is the presence of deoxyribose instead of ribose. An enzyme known as ribonucleotide reductase removes an oxygen molecule from one of the carbons of a ribose sugar. The result is deoxyribose, the base of DNA. This simple change is the only difference between RNA and DNA, while they have evolved different functions over time.
Deoxyribose Structure
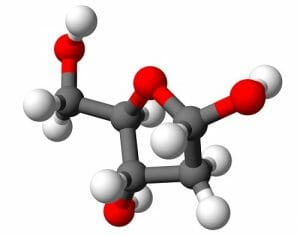
By itself, deoxyribose can exist as a linear molecule or as a five or six-membered ring. Deoxyribose is known as an aldopentose, because it is a five-carbon molecule that contains a carbonyl group at the end of the molecule. In the above image, it is seen as deoxyribofuranose, or as a five membered ring. Substitutions on this ring of a phosphate group and a nucleic acid base will allow deoxyribose to function as the backbone of DNA, as seen in the graphic below.
In DNA, deoxyribose exists as a five-membered ring. As seen in the graphic, deoxyribose has lost an oxygen molecule form one of the carbons in the ring. While this may seem like a simple change, it drastically affects DNA’s resistance to being broken down by hydrolysis. RNA, with the extra oxygen, allows for greater interaction with water molecules. This can lead to hydrolysis of the phosphodiester bonds that link ribose molecules. In comparison, the phosphodiester bonds that link deoxyribose molecules naturally interact less with water, and break down through hydrolysis less. This allows DNA molecules to span generations with only minor fixes.
As a convention, the carbons in a deoxyribose are numbered with primes to differentiate between them. The 1’ carbon (said as “the one prime carbon”) is the carbon that will be bonded to the nitrogenous (nucleic acid) base. The 5’ carbon will be on the opposite side of the ring, and is not part of the ring structure. The 5’ carbon connects to the phosphate group. This phosphate group will then bond to the 3’ carbon of the nucleotide above it, as seen in the graphic. This creates the covalently bonded backbone of DNA. While not pictured, DNA exists as two strands that complement each other, each with deoxyribose based backbones. The pyrimidines and purines interact with each other to form hydrogen bonds with hold the backbones together. During replication, enzymes break these hydrogen bonds to form new strands of DNA that complement each side of the parent strand. New molecules of ribose are attached to nitrogenous bases and phosphate groups before being deoxygenated into deoxyribose bases. The nucleotides can then be added to the growing string of bases which will become an independent DNA molecule.
Related Biology Terms
- Ribose – A pentose molecule bound to 5 oxygen molecules, 1 more than deoxyribose.
- DNA – A nucleic acid polymer made from many individual nucleotides linked by phosphodiester bonds.
- Nucleic Acid Base – The purine or pyrimidine attached to deoxyribose or ribose that create a nucleotide.
- Nucleotide – Deoxyribose or ribose attached to a phosphate group and a nucleic acid base.
Quiz
1. A scientist is experimenting with a substance that forces deoxyribose into its linear form, even when embedded in DNA. What would happen to an organism exposed to this substance?
A. It would reproduce DNA faster, because the DNA would be expanded
B. The DNA would no longer function, and the organism would die
C. The DNA would still function, but could not condense during mitosis
2. DNA can resist damage from hydrolysis due to the lack of an oxygen on the 2’ carbon. Some viruses propagate using only RNA. How can the RNA last through multiple generations even though it uses ribose instead of deoxyribose?
A. After being produced, the RNA is packaged in protein capsules which exclude water.
B. The virus causes water to be excluded from the cell
C. DNA is formed as an intermediate from the RNA, within the cell
3. A scientist adds free phosphate groups, deoxyribose, and all the nucleic acid bases to a beaker. He stirs the beaker with a rod and waits several hours. He tries to analyze the DNA formed in the beaker, but finds there is no DNA, or nucleotides. What is he missing?
A. An organisms to assemble the constituents
B. Electricity
C. Heat, via a blowtorch