Definition
Anaerobic respiration is the type of respiration through which cells can break down sugars to generate energy in the absence of oxygen. This is in contrast to the highly efficient process of aerobic respiration, which relies on oxygen to produce energy.
Molecular oxygen is the most efficient electron acceptor for respiration, due to its high affinity for electrons. However, some organisms have evolved to use other final electron acceptors, and as such, can perform respiration without oxygen.
Overview
Respiration is the process through which the energy stored in fuel is converted into a form that a cell can use. Typically, energy stored in the molecular bonds of a sugar or fat molecule is used to make ATP, by taking electrons from the fuel molecule and using them to power an electron transport chain.
Respiration is crucial to a cell’s survival because if it cannot liberate energy from fuels, it will not have sufficient energy to drive its normal functions. This is why air-breathing organisms die so quickly without a constant supply of oxygen: our cells cannot generate enough energy to stay alive without it.
Instead of oxygen, anaerobic cells use substances such as sulfate, nitrate, sulfur, and fumarate to drive their cellular respiration. Many cells can perform either aerobic or anaerobic respiration, depending on whether oxygen is available.
Anaerobic vs Aerobic Respiration
Similarities
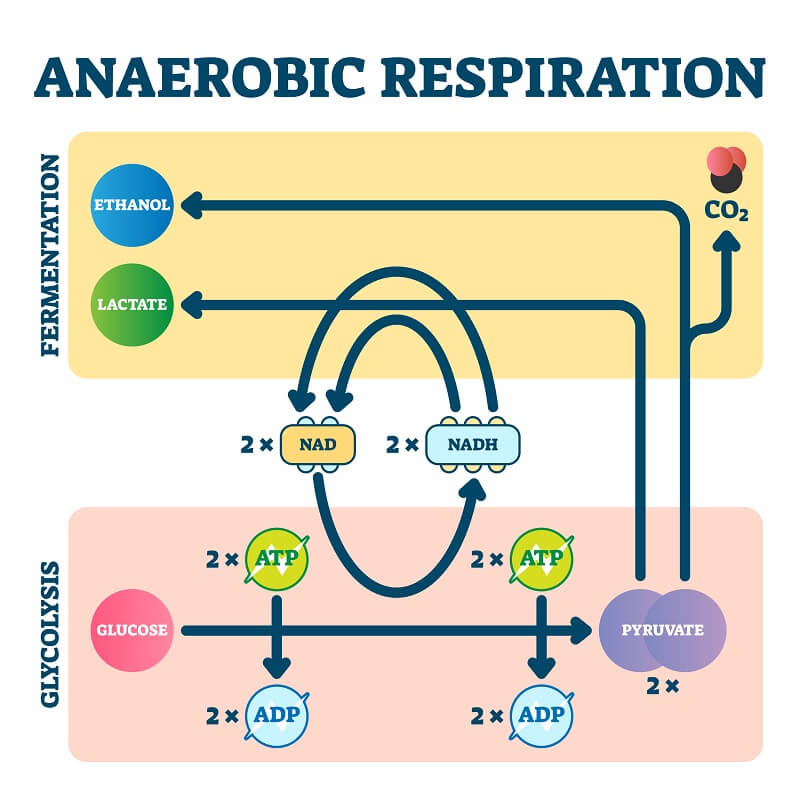
Both aerobic and anaerobic respiration are methods of harvesting energy from a food source, such as fats or sugars. Both processes begin with the splitting of a six-carbon sugar molecule into 2 three-carbon pyruvate molecules in a process called glycolysis. This process consumes two ATP molecules and creates four ATP, for a net gain of two ATP per sugar molecule that is split.
In both aerobic and anaerobic respiration, the two pyruvate molecules are subject to another series of reactions that use electron transport chains to generate more ATP.
It is these reactions that require an electron acceptor – be it oxygen, sulfate, nitrate, etc. – in order to drive them.
Many bacteria and archaea can only perform anaerobic respiration. Many other organisms can perform either aerobic or anaerobic respiration, depending on whether oxygen is present.
Humans and other animals rely on aerobic respiration to stay alive, but can extend their cells’ lives or performance in the absence of oxygen through anaerobic respiration.
Differences
After glycolysis, both the aerobic and anaerobic cells send the two pyruvate molecules through a series of chemical reactions to generate more ATP and extract electrons for use in their electron transport chain.
However, what these reactions are, and where they happen, varies between aerobic and anaerobic respiration
During aerobic respiration, the electron transport chain, and most of the chemical reactions of respiration, occur in the mitochondria. The mitochondria’s system of membranes makes the process much more efficient by concentrating the chemical reactants of respiration together in one small space.
In contrast, anaerobic respiration typically takes place in the cytoplasm. This is because most cells that exclusively carry out anaerobic respiration do not have specialized organelles. The series of reactions is typically shorter in anaerobic respiration and uses a final electron acceptor such as sulfate, nitrate, sulfur, or fumarate instead of oxygen.
Anaerobic respiration also produces less ATP for each sugar molecule digested than aerobic respiration, making it a less efficient method of generating cellular energy. In addition, it produces different waste products – including, in some cases, alcohol!
Cellular Respiration in Different Organisms
Organisms can be classified based on the types of cellular respiration they carry out.
- Obligate aerobes – organisms that cannot survive without oxygen. For example, humans are obligate aerobes.
- Obligate anaerobes – organisms that cannot survive in the presence of oxygen. Certain species of bacteria are obligate anaerobes, such as Clostridium tetani, which causes tetanus.
- Aerotolerant organisms – organisms that can live in the presence of oxygen, but does not use it to grow. For example, the bacterium Streptococcus, which causes Strep throat.
- Facultative aerobes – organisms that can use oxygen to grow, but can also perform anaerobic respiration. For example, Saccharomyces cerevisiae which is the yeast used in brewing.
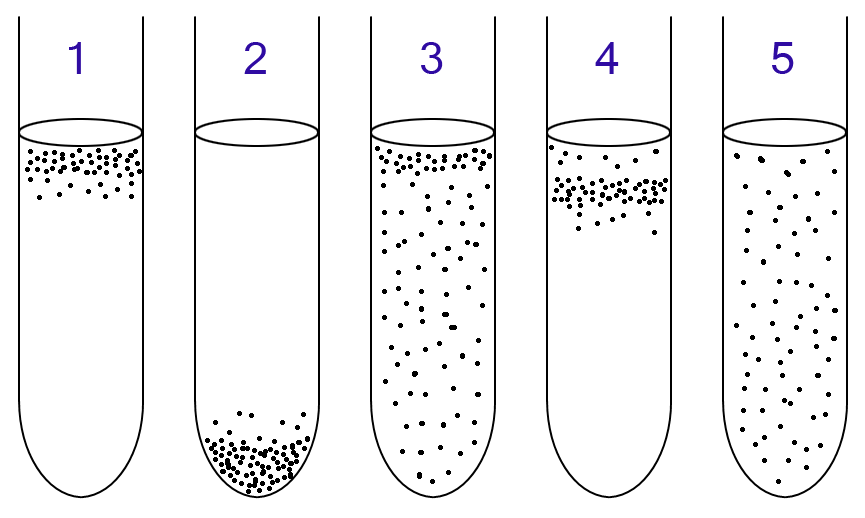
Scientists can classify microbes in this way using a simple experimental set-up with thioglycolate broth. This medium contains a range of oxygen concentrations, producing a gradient. This is because of the presence of sodium thioglycolate, which consumes oxygen, and the continuous supply of oxygen from the air; at the top of the tube, oxygen will be present, and at the bottom, no oxygen will be present.
<h2title=”Types”>Types of Anaerobic Respiration
The types of anaerobic respiration are as varied as its electron acceptors. Important types of anaerobic respiration include:
- Lactic acid fermentation – In this type of anaerobic respiration, glucose is split into two molecules of lactic acid to produce two ATP. It occurs in certain types of bacteria and some animal tissues, such as muscle cells
- Alcoholic fermentation – In this type of anaerobic respiration, glucose is split into ethanol or ethyl alcohol. This process also produces two ATP per sugar molecule. This occurs in yeast and even in some types of fish, such as goldfish.
- Other types of fermentation – Other types of fermentation are performed by some bacteria and archaea. These include propionic acid fermentation, butyric acid fermentation, solvent fermentation, mixed acid fermentation, butanediol fermentation, Stickland fermentation, acetogenesis, and methanogenesis.
Anaerobic Respiration Equations
The equations for the two most common types of anaerobic respiration are:
• Lactic acid fermentation:
C6H12O6 (glucose)+ 2 ADP + 2 pi → 2 lactic acid + 2 ATP
• Alcoholic fermentation:
C6H12O6 (glucose) + 2 ADP + 2 pi → 2 C2H5OH (ethanol) + 2 CO2 + 2 ATP
Examples of Anaerobic Respiration
Sore Muscles and Lactic Acid
During intense exercise, our muscles use oxygen to produce ATP faster than we can supply it.
When this happens, muscle cells can perform glycolysis faster than they can supply oxygen to the mitochondrial electron transport chain.
The result is that anaerobic respiration and lactic acid fermentation occurs within our cells – and after prolonged exercise, the built-up lactic acid can make our muscles sore!
Yeasts and Alcoholic Drinks

Alcoholic drinks such as wine and whiskey are typically produced by bottling yeasts – which perform alcoholic fermentation – with a solution of sugar and other flavoring compounds.
Yeasts can use complex carbohydrates including those found in potatoes, grapes, corn, and many other grains, as sources of sugar to carry out cellular respiration.
Putting the yeast and its fuel source in an airtight bottle ensures that there will not be enough oxygen around, and thus the yeast will convert to anaerobic respiration. This produces alcohol.
Alcohol is actually toxic to the yeasts that produce it – when alcohol concentrations become high enough, the yeast will begin to die.
For that reason, it is not possible to brew wine or a beer that has greater than 30% alcohol content. However, the process of distillation, which separates alcohol from other components of the brew, can be used to concentrate the alcohol and produce spirits such as vodka.
Methanogenesis and Dangerous Homebrews
Unfortunately, alcoholic fermentation isn’t the only kind of fermentation that can happen in plant matter. A different alcohol, called methanol, can be produced from the fermentation of cellulose. This can cause methanol poisoning.
The dangers of “moonshine” – cheap, homebrewed alcohol which often contains high amounts of methanol due to poor brewing and distillation processes – were advertised in the 20th century during prohibition.
Death and nerve damage from methanol poisoning is still an issue in areas where people try to brew alcohol cheaply. So, if you’re going to become a brewer, make sure you do your homework!
Swiss Cheese and Propionic Acid
Propionic acid fermentation gives Swiss cheese its distinctive flavor. The holes in Swiss cheese are actually made by bubbles of carbon dioxide gas released as a waste product of a bacteria that uses propionic acid fermentation.

After the implementation of stricter sanitation standards in the 20th century, many producers of Swiss cheese were puzzled to find that their cheese was losing its holes – and its flavor.
The culprit was discovered to be a lack of a specific bacteria which produce propionic acid. Throughout the ages, this bacteria had been introduced as a contaminant from the hay the cows ate. But after stricter hygiene standards were introduced, this was not happening anymore!
This bacteria is now added intentionally during production to ensure that Swiss cheese stays flavorful and retains its instantly recognizable holey appearance.
Vinegar and Acetogenesis
Bacteria that perform acetogenesis are responsible for the making of vinegar, which consists mainly of acetic acid.
Vinegar actually requires two fermentation processes, because the bacteria that make acetic acid require alcohol as fuel!
As such, vinegar is first fermented into an alcoholic preparation, such as wine. The alcoholic mixture is then fermented again using the acetogenic bacteria.
Quiz
