Molecule Definition
A molecule is two or more atoms bonded together to form a single chemical entity. Each atom carries a certain number of electrons that orbit around the nucleus. The nucleus consists of protons and neutrons, of different numbers in different elements. The electrons that orbit the nucleus exist in various clouds, or valence shells. These shells prefer to have specific numbers of electons, depending on the shell. Sometimes, one atom will give away electrons to another atom. These atoms both change in electrical charge and become ions. One will be positive and one will be negative. These opposite electrical effects attract each other and form ionic bonds. These bonds to not make a molecule, and the ions can be easily separated. However, sometimes atom share electrons.
When two atoms share an electron, or multiple electrons, a strong bond is formed between them as the electron passes from one nucleus to the other and back. This electron activity ties the two atoms together. Molecules can form single bond, double bonds, triple bonds, and even more, depending on how many electrons they are sharing. Sharing an electron is known as a covalent bond and is very important in biology. Not only are covalent bond stronger that ionic bonds, but they store more energy. Organisms can use this to their advantage by storing energy in chemical bonds. It also means that the covalent bonds in food must be broken apart to gain energy. This is why our bodies have millions of enzymes, bacteria, and fungi that function together to break the many covalent bonds present in our food and release the energy.
Examples of Molecule
Carbon-Based Molecules
Carbon is probably the most important element for all living organisms. Carbon has a unique ability to form 4 covalent bonds, which can lead to long chains of molecules. All organic molecules contain carbon, and the ability to manipulate carbon bonds was probably a very early development in the evolution of life. All of the types of molecules described below contain carbon, with a wide variety of other atoms covalently bonded to the carbon. Carbon, when it forms double bonds with other carbon atoms, can rotate around the bond. This can create molecule that are flexible, and vary in shape. The wide variety of differently shaped carbon molecules in the biological world produces unique interactions.
Adenosine Triphosphate (ATP)
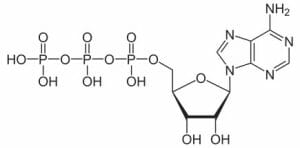
A molecule that nearly every organism uses is adenosine triphosphate or ATP. Adenosine is molecule of multiple carbon rings, as represented by the right side of the molecule below. The left side is a chain of phosphate groups, which are a phosphorus atoms covalently bonded to oxygen atoms. When the bonds between theses phosphate groups are broken, energy is released. Usually ATP functions as a coenzyme, transferring the energy from the bond to an enzyme, which can use the energy to speed a chemical reaction. Two molecules are present after the break, a free-floating phosphate group and adenosine diphosphate or ADP. Through the processes of glycolysis (the breakdown of glucose) and respiration (the use of oxygen to further break down glucose), ATP is produced, which can then be used for energy in other cellular processes.
Types of Biological Molecules
A molecule may have very different properties than the atoms that make it up. For instance, sugar is a combination of carbon, oxygen, and hydrogen. Carbon, as you’ve seen at the end of a fire, is a gray-dusty substance. Oxygen and hydrogen are both gases. Somehow, when combined together with covalent bonds, strings of carbon with oxygen and hydrogen become a sweet and energy-rich nutrient that many animals rely on for survival. In biology, there are many molecules that animals produce, but they only come in a few types.
Proteins
One of the most important types of molecule produced by cells is protein. A protein molecule is a polymer. This means it was formed from many smaller molecules, known as monomers. These molecules are called amino acids. The DNA of every organism codes for specific sequences of amino acids. The proper amino acids are strung together, and the complex interactions between the amino acids causes they string to fold. These fold lead to more complex structures. The structure of a protein allows it to function in different ways.
Cells use protein molecules in a wide variety of tasks. They can be used as enzymes to catalyze specific reactions. They can form antibodies, as part of an organism’s immune defenses. Some proteins simply store amino acids, for use later. There are proteins embedded in the cell membranes, which allow ions and other molecules to pass through the membranes. On nerve cells, proteins are used to receive signals sent by others nerves, thereby passing the signal along. In muscle cells, proteins are responsible for causing the muscles to contract. Still other proteins are used simply as structural support. The list of functions cells use protein molecules for is enormous.
Lipids
Another important class of molecule is the lipid class. Lipids are molecules that don’t mix well with water, called hydrophobic. Oftentimes, the bonds in the molecules of a lipid to not create charges, and are nonpolar. These nonpolar molecules do not like to mix with water, a very polar molecule. Lipids are also polymers, and are created from two smaller molecules, glycerol and a fatty acid. These lipid molecules store a lot of energy, and are often used in fat cells, to store energy for an organism. Sometimes, a hydrophilic, or water-loving, phosphate head is attached to lipid molecules. This creates a phospholipid. Many phospholipids can be put together to create cell membranes. Sometimes, lipids can become steroids, or chemicals that make cells respond in different ways. One of these, cholesterol, can influence how stiff cell membranes are, which can in turn influence how stiff arteries and veins are. This is one reason why doctors recommend lowering cholesterol, so tissues can have the right texture.
Carbohydrates
While proteins and lipids provide structure, support, and enzyme functions, carbohydrates are responsible mostly for energy. Most animals process some sort of sugar to allow their cells to function. Plants often store these sugars as more complex carbohydrates, like starches. Individual sugars are known as monosaccharides while multiple sugars connected are called polysaccharides. Plants sometimes use these carbohydrate molecules for other functions, such as structure. The main structural carbohydrate plants use is cellulose, which they use to build cell walls around their cells. By putting pressure on a water-filled vacuole inside the cell, the cellulose molecules are pushed together and become rigid.
As an energy molecule, plants create glucose through photosynthesis. By utilizing the energy of light plants can store energy in the bonds of glucose. Although glucose is an easy molecule to get energy from, it is not convenient to store. Instead, plants combine glucose molecules together to form bigger polysaccharides, which can be stacked and stored in specialized cells for use later. Animals are well aware of this fact, and herbivores can survive on only the glucose and other carbohydrates present in plant mater. In fact, even humans can thrive on an herbivorous diet because plants have all the carbohydrates and protein a person needs.
Nucleic Acids
The most important molecule of life, DNA, is made from intertwined strings of nucleic acids. Nucleic acids are molecules that alone mean nothing, but when connected in a series hold information. The information they carry can be “read” by certain proteins that work together to translate the codons of DNA into strings of amino acids, which fold into functional proteins. This process of creating proteins from information contained in molecules is known as biosynthesis and is the basis of all life. Organisms can copy their information molecules and pass their genetics on to their offspring. The beginnings of life probably started with only one or two of these self-replicating molecules, and over billions of years has expanded (and contracted) into the diversity we see today.
Related Biology Terms
- Valence Shell – The electron shell of atoms that interacts with other atoms.
- Covalent Bond – A bond between atoms in which the electrons are shared.
- Ionic Bond – A bond between atoms caused by electrical attraction between atoms.
- Atom – A single unit of an element, or a nucleus of protons and neutrons surrounded by electrons.
Quiz
1. Which of the following is NOT a molecule?
A. H2O
B. Cl–
C. O2
2. Table salt consists of two ions, Na+ and Cl– that exist in a matrix. The ions do not bond together, but are attracted to each other and form table salt, or NaCl. Is NaCl a molecule?
A. Yes
B. No
C. Only when in the matrix
3. Polymers are monomers bonded together. Monomers are an example of what?
A. Molecules
B. Atoms
C. Nuclei