Carbonyl Group Definition
A carbonyl group is a functional group characterized by a carbon atom double bonded to an oxygen, found within a larger carbon-based molecule. The electronegativity of oxygen creates a resonance hybrid structure in which the electrons are continuously redistributed. This allows the molecule to participate in more reactions.
Carbonyl Group Overview
The power of a carbonyl group comes from the difference in electronegativity between carbon and oxygen. Oxygen is much more electronegative, which means it tends to pull electrons away from the carbon. As such, the carbon has more room for electrons from other atoms within the molecule. This allows the overall molecule to take on several different structures, known as resonance hybrid structures.
As the molecule interacts with the huge variety of other molecules within its environment, the resonant structures of the molecule can interact with a wide variety of the other molecules present. Without the disruption of the electron structure caused by a carbonyl group, a mostly-carbon molecule could not interact with as many other molecules.
Within biology, a carbonyl group within a molecule allows it to undergo the many reactions necessary to maintain life. Many common biological molecules contain a carbonyl group, which allows the cell the ability to create new molecules and modify the molecule with a number of other functional groups.
Carbonyl Group Structure
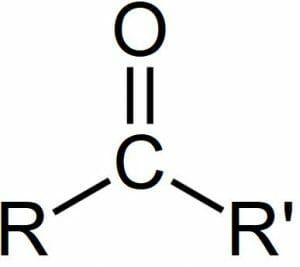
A carbonyl group can be seen in the image below, with R and R’ representing more carbons in a larger chain which the central carbonyl group is attached to. The carbonyl group may be attached to more carbons, as in the image below. Or, a carbonyl group may have single atoms attach, as is the case with carbon dioxide.
While this is a relatively simple structure, it leads to a number of chemical reactions that may take place. This is largely due to the propensity of the oxygen atom to horde electrons within the bonds. This creates a point of polarity, which can interact with a number of other atoms and molecules.
Carbonyl Group Function
Destabilizing Bonds within a Molecule
The carbonyl group serves the functional role of destabilizing the bonds within the carbon chain. The electronegative oxygen atom tends to attract more electrons than the carbon it is bonded to within the carbonyl group. As such, the carbon within the carbonyl group will become more positive, as it loses the electrical influence of the negative electrons that are drawn toward the oxygen. The carbon is now called a carbocation because it has a slight positive charge. This slightly positive charge on the carbon of the carbonyl group sets up a wide variety of possible reactions that result from the change in electron distribution.
Carbonyl Group as an Electron Sink
Besides creating a slightly positive carbocation within the carbonyl group, the influence of the oxygen atom and resulting carbocation can stabilize the formation of a carbanion intermediate in one of the adjacent carbons. The carbonyl group is known informally as an electron sink, which is able to “soak up” extra electrons in the molecule. These extra electrons will flow between the carbons and the oxygen, temporarily creating double bonds which fade to more electrically negative and positive areas.
This destabilization allows for a number of important reactions. Many reactions that form carbon-carbon bonds are started with the creation of nucleophiles and electrophiles with carbonyl groups. The carbon of the carbonyl group is able to accept electrons to form carbon-carbon bonds as the electrophile, while a carbanion intermediate (created by another carbonyl group) can serve as the nucleophile and donate the electrons.
Utilization through Catalysts
Biochemical processes in organisms manipulate the activity of the carbonyl group through metals and acids in the environment reactions will take place. Various acids and metals will react with the oxygen, lowering its electronegativity. In turn, the oxygen will take less of the electrons from the carbon molecule it is attached to. This favors the formation of the negative carbanion intermediate instead of a carbon-carbon double-bond and leads to the formation of new carbon-carbon bonds. Similarly, imine groups cause similar delocalization of negative charges on carbons and can facilitate the same kinds of reactions.
Common Chemicals with a Carbonyl Group
Several commonly encountered chemicals are formed with carbonyl groups. Aldehydes and ketones, for example, both have a carbonyl group. Aldehydes have the carbonyl group at the end of a series of bonded carbons. Ketones, on the other hand, have the carbonyl group in the middle of several bonded carbon atoms. This difference creates highly variable bonding conditions within the molecules, which influences how they interact with other substances.
Further, a common component used to create amino acids is carboxylic acid. This chemical has a carbonyl group attached directly to a hydroxide group, which allows for the formation of proteins. The carbonyl group creates the necessary conditions for the hydroxide group to be replaced with a bond to the N terminus of another amino acid. This creates a covalent bond between the two amino acids which is very hard to break.
The simplest carbonyl group is likely carbon dioxide, which is simply a carbonyl group with a substituted oxygen atom. This molecule is created as a byproduct of cellular respiration, and it is heavily involved in the carbon cycle.
Quiz